Almestrone
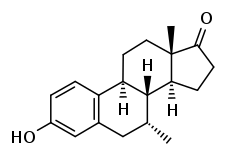 | |
| Clinical data | |
|---|---|
| Other names | Ba 38372; Ciba 38372; 7α-Methylestrone |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.212.018 |
| Chemical and physical data | |
| Formula | C19H24O2 |
| Molar mass | 284.399 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Almestrone (INN) (developmental code names Ba 38372, Ciba 38372), also known as 7α-methylestrone, is a synthetic, steroidal estrogen which was synthesized in 1967 but was never marketed.[1] It is used as a precursor in the synthesis of several highly active steroids.[2][3]
See also
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 900–. ISBN 978-1-4757-2085-3.
- ↑ Krause N, Aksin-Artok Ö (2011). "Copper-Mediated and Copper-Catalyzed Addition and Substitution Reactions of Extended Multiple Bond Systems". PATai's Chemistry of Functional Groups. doi:10.1002/9780470682531.pat0450. ISBN 9780470682531.
- ↑ Morais GR, Yoshioka N, Watanabe M, Mataka S, Oliveira CD, Thiemann T (2006). "C7-Substituted Estranes and Related Steroids". Mini-Reviews in Organic Chemistry. 3 (3): 229–251. doi:10.2174/1570193X10603030229. ISSN 1570-193X.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.