Levormeloxifene
 | |
| Clinical data | |
|---|---|
| Other names | Levomeloxifene; 6720-CDRI; NNC-460020 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
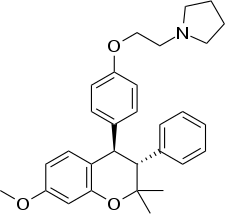
| Formula | C30H35NO3 |
| Molar mass | 457.614 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Levormeloxifene (INN; developmental code names 6720-CDRI, NNC-460020) is a selective estrogen receptor modulator (SERM) which was being developed as an alternative to estrogen replacement therapy for the treatment and prevention of postmenopausal bone loss but did not complete development and hence was never marketed.[1] The development was stopped because of a high incidence of gynecological side effects during clinical trials.[2] Levormeloxifene is the levorotatory enantiomer of ormeloxifene, which, in contrast, has been marketed, though rather as a hormonal contraceptive.
See also
References
- ↑ "Levormeloxifene - AdisInsight".
- ↑ Ravn P, Nielsen TF, Christiansen C (2006). "What can be learned from the levormeloxifene experience?". Acta Obstet Gynecol Scand. 85 (2): 135–42. doi:10.1080/00016340500345691. PMID 16532904. S2CID 12872469.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.