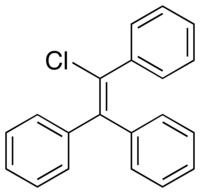
Triphenylchloroethylene
 | |
| Clinical data | |
|---|---|
| Trade names | Gynosone, Oestrogyl |
| Other names | TPCE; Triphenylchlorethylene; Chlorotriphenylethylene; Phenylstilbene chloride |
| Drug class | Nonsteroidal estrogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C20H15Cl |
| Molar mass | 290.79 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Triphenylchloroethylene (TPCE; brand names Gynosone, Oestrogyl), or triphenylchlorethylene, also known as chlorotriphenylethylene or as phenylstilbene chloride, is a synthetic nonsteroidal estrogen of the triphenylethylene group that was marketed in the 1940s for the treatment of menopausal symptoms, vaginal atrophy, lactation suppression, and all other estrogen-indicated conditions.[1][2][3]
The estrogenic effects of triphenylethylene, the parent compound of triphenylchloroethylene, were discovered in 1937.[4] Triphenylchloroethylene was first reported in 1938 and was found to have 20 to 100 times the estrogenic activity of the relatively weak triphenylethylene, a potentiation of effect that was afforded by its halogen substituent.[2][5] The drug has a relatively long duration of action when administered via subcutaneous injection but a duration similar to that of diethylstilbestrol or estradiol benzoate when administered orally.[2][6][7] Along with diethylstilbestrol and triphenylmethylethylene, triphenylchloroethylene was studied in 1944 by Sir Alexander Haddow for the treatment of breast cancer and was found to be significantly effective, and this is historically notable in that it was the first time that high-dose estrogens were found to be effective in the treatment of breast cancer.[8][9]
Chlorotrianisene, or tri-p-anisylchloroethylene (TACE), is a potent marketed estrogen and a derivative of triphenylchloroethylene in which each of the three phenyl rings has been substituted with a 4-methoxy group.[5][10] Estrobin, or DBE, is a related, never-marketed estrogen in which there is a bromine in place of the chlorine and two of the three phenyl rings have ethoxy groups.[5] Broparestrol, or BDPE is a marketed selective estrogen receptor modulator (SERM) that has a bromine in place of the chlorine of triphenylchloroethylene and a 4-ethyl group on one of the phenyl rings. The SERM clomifene is also a derivative of triphenylchloroethylene.[11]
See also
References
- ↑ Martin Negwer; Hans-Georg Scharnow (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. pp. 1861–1862. ISBN 978-3-527-30247-5.
C20H15Cl 18084-97-4 Chlorotriphenylethene = Triphenylchloroethylene = Phenylstilbene chloride = 1,1',1"-(1-Chloro-1-ethenyl-2-ylidene)tris[benzene] (•) S Gynosone, Oestrogyl, Phenylstilbene chloride U Synthetic estrogen
- 1 2 3 EMMENS CW (1947). "Halogen-substituted oestrogens related to triphenylethylene". J. Endocrinol. 5 (3): 170–3. doi:10.1677/joe.0.0050170. PMID 20259249.
- ↑ "Advertisement" (PDF). Proc R Soc Med. 39 (11). Sep 1946. PMC 2181944.
- ↑ Jie Jack Li (3 April 2009). Triumph of the Heart: The Story of Statins. Oxford University Press, USA. pp. 33–. ISBN 978-0-19-532357-3.
- 1 2 3 SOLMSSEN UV (1945). "Synthetic estrogens and the relation between their structure and their activity". Chem. Rev. 37 (3): 481–598. doi:10.1021/cr60118a004. PMID 21013428.
- ↑ Virgil Craig Jordan (1986). Estrogen/antiestrogen Action and Breast Cancer Therapy. Univ of Wisconsin Press. pp. 23–. ISBN 978-0-299-10480-1.
- ↑ Philipp Y. Maximov; Russell E. McDaniel; V. Craig Jordan (23 July 2013). Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Science & Business Media. pp. 4–. ISBN 978-3-0348-0664-0.
- ↑ V Craig Jordan (27 May 2013). Estrogen Action, Selective Estrogen Receptor Modulators and Women's Health: Progress and Promise. World Scientific. pp. 42–. ISBN 978-1-84816-959-3.
- ↑ D. J. Th. Wagener (13 July 2009). The History of Oncology. Bohn Stafleu van Loghum. pp. 189–. ISBN 978-90-313-6143-4.
- ↑ Gautam Allahbadia (2006). Contemporary Perspectives on Assisted Reproductive Technology. Elsevier India. pp. 18–. ISBN 978-81-8147-782-8.
- ↑ David K. Gardner; Ariel Weissman; Colin M Howles; Zeev Shoham (27 June 2012). Textbook of Assisted Reproductive Techniques Fourth Edition: Volume 2: Clinical Perspectives. CRC Press. pp. 51–. ISBN 978-1-84184-972-0.