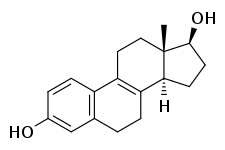
8,9-Dehydroestradiol
 | |
| Clinical data | |
|---|---|
| Other names | Δ8-Estradiol; Δ8-17β-Estradiol; Estra-1,3,5(10),8-tetraen-17β-ol-3-one |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C18H22O2 |
| Molar mass | 270.372 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
8,9-Dehydroestradiol, or Δ8-17β-estradiol, also known as estra-1,3,5(10),8-tetraen-17β-ol-3-one, is a naturally occurring steroidal estrogen found in horses which is closely related to equilin, equilenin, and estradiol, and, as the 3-sulfate ester sodium salt, is a minor constituent of conjugated estrogens (Premarin).[1] It is also an important active metabolite of 8,9-dehydroestrone, analogously to conversion of estrone or estrone sulfate into estradiol.[2][3]
See also
References
- ↑ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 751–. ISBN 978-1-4511-4847-3.
- ↑ Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ↑ Bhavnani BR, Nisker JA, Martin J, Aletebi F, Watson L, Milne JK (2000). "Comparison of pharmacokinetics of a conjugated equine estrogen preparation (premarin) and a synthetic mixture of estrogens (C.E.S.) in postmenopausal women". J. Soc. Gynecol. Investig. 7 (3): 175–83. doi:10.1016/s1071-5576(00)00049-6. PMID 10865186.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.