Etacstil
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
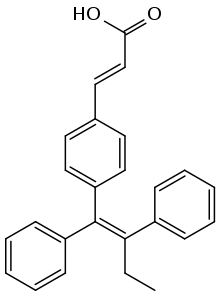
| Formula | C25H22O2 |
| Molar mass | 354.449 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Etacstil (developmental code names GW-5638, DPC974) is an orally active, nonsteroidal, combined selective estrogen receptor modulator (SERM) and selective estrogen receptor degrader (SERD) that was developed for the treatment of estrogen receptor-positive breast cancer.[1][2][3] It was shown to overcome antiestrogen (tamoxifen, aromatase inhibitor, fulvestrant) resistance in breast cancer by altering the shape of the estrogen receptor, thus exhibiting SERD properties.[4][5][6][7][8] Etacstil is a tamoxifen derivative and one of the first drugs to overcome tamoxifen-resistance. It is the predecessor of GW-7604,[3][9][10] of which etacstil is a prodrug (GW-7604 being the 4-hydroxy metabolite of etacstil).[11] This is analogous to the case of tamoxifen being a prodrug of afimoxifene (4-hydroxytamoxifen).[11]
Etacstil was developed in the early 1990s by Duke University, Glaxo Wellcome, and later, Dupont.[12][13] In 2001, Bristol Myers-Squibb (BMS) acquired Dupont, and for non-scientific, corporate reasons, closed the trial and abandoned the release of etacstil and its metabolite GW-7604.[6][9][12]
After many dormant years, a recent resurgence of interest in SERDs has led to the development of brilanestrant, a structural analogue of etacstil.[9]
See also
- Bazedoxifene
- Elacestrant
- Timeline of cancer treatment development
References
- ↑ Kelloff, Gary J.; Hawk, Ernest T.; Sigman, Caroline C. (2008-08-17). Cancer Chemoprevention: Volume 2: Strategies for Cancer Chemoprevention. ISBN 9781592597680.
- ↑ "T GW 5638 Profile".
- 1 2 Becnel, LB; Darlington, YF; Orechsner, S.; Easton-Marks, J.; Watkins, CA; McOwiti, A.; Kankanamge, WH; Dehart, M.; Silva, CM; Margolis, RN; McKenna, NJ. "Nuclear Receptor Signaling Atlas". doi:10.1621/B4A9CIQ78V.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Antiestrogen GW5638 induces a unique structural change in the ER. The biological significance of this conformational change was revealed in studies that demonstrated that tamoxifen-resistant breast tumor explants are not cross-resistant to GW5638. Because of these properties, this drug is currently being developed as a potential therapeutic for tamoxifen-resistant breast cancers.Connor CE, Norris JD, Broadwater G, Willson TM, Gottardis MM, Dewhirst MW, McDonnell DP (2001). "Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational change in the estrogen receptor". Cancer Res. 61 (7): 2917–22. PMID 11306468.
- ↑ "GW5638 uniquely alters the shape of the estrogen receptor ".
- 1 2 "Tamoxifen-like drug suggests new ways to selectively block estrogen".
- ↑ Dardes RC, O'Regan RM, Gajdos C, Robinson SP, Bentrem D, De Los Reyes A, Jordan VC (2002). "Effects of a new clinically relevant antiestrogen (GW5638) related to tamoxifen on breast and endometrial cancer growth in vivo". Clin. Cancer Res. 8 (6): 1995–2001. PMID 12060645.
- ↑ Tong, Sheng; Chen, Qing; Shan, Si-Qing; Dewhirst, Mark W.; Yuan, Fan (2006). "Quantitative comparison of the inhibitory effects of GW5638 and tamoxifen on angiogenesis in the cornea pocket assay". Angiogenesis. 9 (2): 53–58. doi:10.1007/s10456-006-9029-x. PMID 16622786. S2CID 35414830.
- 1 2 3 Wardell SE, Nelson ER, Chao CA, Alley HM, McDonnell DP (2015). "Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader". Endocr Relat Cancer. 22 (5): 713–24. doi:10.1530/ERC-15-0287. PMC 4545300. PMID 26162914.
- ↑ Bentrem D, Dardes R, Liu H, MacGregor-Schafer J, Zapf J, Jordan V (2001). "Molecular mechanism of action at estrogen receptor alpha of a new clinically relevant antiestrogen (GW7604) related to tamoxifen". Endocrinology. 142 (2): 838–46. doi:10.1210/endo.142.2.7932. PMID 11159857.
- 1 2 Bentrem D, Dardes R, Liu H, MacGregor-Schafer J, Zapf J, Jordan V (2001). "Molecular mechanism of action at estrogen receptor alpha of a new clinically relevant antiestrogen (GW7604) related to tamoxifen". Endocrinology. 142 (2): 838–46. doi:10.1210/endo.142.2.7932. PMID 11159857.
- 1 2 "how breast cancer drugs are developed". 17 June 2013.
- ↑ Willson TM, Henke BR, Momtahen TM, Charifson PS, Batchelor KW, Lubahn DB, Moore LB, Oliver BB, Sauls HR, Triantafillou JA (1994). "a non-steroidal estrogen with functional selectivity for bone over uterus in rats". J Med Chem. 37 (11): 1550–2. doi:10.1021/jm00037a002. PMID 8201587.