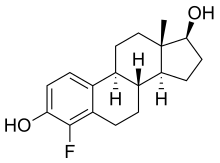
4-Fluoroestradiol
 | |
| Clinical data | |
|---|---|
| Other names | 4-FE2; 4-F-17β-E2; 4-Fluoro-17β-estradiol; 4-Fluoroestra-1,3,5-(10)-triene-3,17β-diol; NSC-94528 |
| Drug class | Estrogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H23FO2 |
| Molar mass | 290.378 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
4-Fluoroestradiol (4-FE2) is a synthetic estrogen and a derivative of estradiol which was never marketed.[1] It is specifically the 4-fluoro analogue of estradiol.[1] 4-Fluoroestradiol has 180 ± 43% of the affinity of estradiol for the estrogen receptor of rat uterine cytosol and shows potent uterotrophic activity similar to that of estradiol in mice and rats.[1][2] It has been labeled with fluorine-18 (18F) for potential use in medical imaging.[3][4][5]
| Estrogen | ER RBA (%) | Uterine weight (%) | Uterotrophy | LH levels (%) | SHBG RBA (%) |
|---|---|---|---|---|---|
| Control | – | 100 | – | 100 | – |
| Estradiol | 100 | 506 ± 20 | +++ | 12–19 | 100 |
| Estrone | 11 ± 8 | 490 ± 22 | +++ | ? | 20 |
| Estriol | 10 ± 4 | 468 ± 30 | +++ | 8–18 | 3 |
| Estetrol | 0.5 ± 0.2 | ? | Inactive | ? | 1 |
| 17α-Estradiol | 4.2 ± 0.8 | ? | ? | ? | ? |
| 2-Hydroxyestradiol | 24 ± 7 | 285 ± 8 | +b | 31–61 | 28 |
| 2-Methoxyestradiol | 0.05 ± 0.04 | 101 | Inactive | ? | 130 |
| 4-Hydroxyestradiol | 45 ± 12 | ? | ? | ? | ? |
| 4-Methoxyestradiol | 1.3 ± 0.2 | 260 | ++ | ? | 9 |
| 4-Fluoroestradiola | 180 ± 43 | ? | +++ | ? | ? |
| 2-Hydroxyestrone | 1.9 ± 0.8 | 130 ± 9 | Inactive | 110–142 | 8 |
| 2-Methoxyestrone | 0.01 ± 0.00 | 103 ± 7 | Inactive | 95–100 | 120 |
| 4-Hydroxyestrone | 11 ± 4 | 351 | ++ | 21–50 | 35 |
| 4-Methoxyestrone | 0.13 ± 0.04 | 338 | ++ | 65–92 | 12 |
| 16α-Hydroxyestrone | 2.8 ± 1.0 | 552 ± 42 | +++ | 7–24 | <0.5 |
| 2-Hydroxyestriol | 0.9 ± 0.3 | 302 | +b | ? | ? |
| 2-Methoxyestriol | 0.01 ± 0.00 | ? | Inactive | ? | 4 |
| Notes: Values are mean ± SD or range. ER RBA = Relative binding affinity to estrogen receptors of rat uterine cytosol. Uterine weight = Percentage change in uterine wet weight of ovariectomized rats after 72 hours with continuous administration of 1 μg/hour via subcutaneously implanted osmotic pumps. LH levels = Luteinizing hormone levels relative to baseline of ovariectomized rats after 24 to 72 hours of continuous administration via subcutaneous implant. Footnotes: a = Synthetic (i.e., not endogenous). b = Atypical uterotrophic effect which plateaus within 48 hours (estradiol's uterotrophy continues linearly up to 72 hours). Sources: See template. | |||||
See also
References
- 1 2 3 Martucci CP (July 1983). "The role of 2-methoxyestrone in estrogen action". J. Steroid Biochem. 19 (1B): 635–8. doi:10.1016/0022-4731(83)90229-7. PMID 6310247.
- ↑ Longcope C, Rafkind I, Arunachalam T, Caspi E (September 1983). "Biological activities of 4-fluoro estrogen analogues". J. Steroid Biochem. 19 (3): 1325–8. doi:10.1016/0022-4731(83)90158-9. PMID 6621038.
- ↑ Eakins MN, Palmer AJ, Waters SL (November 1979). "Studies in the rat with 18F-4-fluoro-oestradiol and 18F-4-fluoro-oestrone as potential prostate scanning agents: comparison with 125I-2-iodo-oestradiol and 125I-2,4-di-iodo-oestradiol". Int J Appl Radiat Isot. 30 (11): 695–700. doi:10.1016/0020-708x(79)90111-x. PMID 544526.
- ↑ Cummins CH (June 1993). "Radiolabeled steroidal estrogens in cancer research". Steroids. 58 (6): 245–59. doi:10.1016/0039-128x(93)90069-y. PMID 8212070. S2CID 29080385.
- ↑ Jasem, Yosef Al; Thiemann, Thies; Gano, Lurdes; Oliveira, Maria Cristina (2016). "Fluorinated steroids and their derivatives". Journal of Fluorine Chemistry. 185: 48–85. doi:10.1016/j.jfluchem.2016.03.009. ISSN 0022-1139.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.