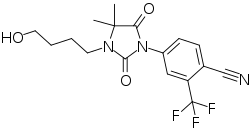
RU-58841
 | |
| Clinical data | |
|---|---|
| Other names | PSK-3841; HMR-3841 |
| Drug class | Nonsteroidal antiandrogen |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H18F3N3O3 |
| Molar mass | 369.344 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
RU-58841, also known as PSK-3841 or HMR-3841, is a nonsteroidal antiandrogen (NSAA) which was initially developed in the 1980s by Roussel Uclaf, the French pharmaceutical company from which it received its name. It was formerly under investigation by ProStrakan (previously ProSkelia and Strakan) for potential use as a topical treatment for androgen-dependent conditions including acne, pattern hair loss,[1] and excessive hair growth.[2][3][4][5] The compound is similar in structure to the NSAA RU-58642 but contains a different side-chain.[6] These compounds are similar in chemical structure to nilutamide,[7] which is related to flutamide, bicalutamide, and enzalutamide, all of which are NSAAs similarly.[8] RU-58841 can be synthesized either by building the hydantoin moiety or by aryl coupling to 5,5-dimethylhydantoin.[9]
RU-58841 produces cyanonilutamide (RU-56279) and RU-59416 as metabolites in animals.[10] Cyanonilutamide has relatively low affinity for the androgen receptor but shows significant antiandrogenic activity in animals.[10] RU-59416 has very low affinity for the androgen receptor.[10]
See also
References
- ↑ "RU58841 studies and information". Anagen.
- ↑ PSK-3841 (HMR-3841, RU-58841) - AdisInsight
- ↑ Battmann T, Bonfils A, Branche C, Humbert J, Goubet F, Teutsch G, Philibert D (January 1994). "RU 58841, a new specific topical antiandrogen: a candidate of choice for the treatment of acne, androgenetic alopecia and hirsutism". J. Steroid Biochem. Mol. Biol. 48 (1): 55–60. doi:10.1016/0960-0760(94)90250-X. PMID 8136306. S2CID 31052540.
- ↑ Münster U, Nakamura C, Haberland A, Jores K, Mehnert W, Rummel S, Schaller M, Korting HC, Zouboulis C, Blume-Peytavi U, Schäfer-Korting M (January 2005). "RU 58841-myristate--prodrug development for topical treatment of acne and androgenetic alopecia". Pharmazie. 60 (1): 8–12. PMID 15700772.
- ↑ "RU58841 for Hair Loss – Underused Finasteride Alternative?". Hairverse. 2019-03-10. Retrieved 2020-07-14.
- ↑ Van Dort ME, Jung YW (April 2001). "Synthesis and structure-activity studies of side-chain derivatized arylhydantoins for investigation as androgen receptor radioligands". Bioorg. Med. Chem. Lett. 11 (8): 1045–7. doi:10.1016/s0960-894x(01)00146-9. PMID 11327585.
- ↑ Poulos GA, Mirmirani P (February 2005). "Investigational medications in the treatment of alopecia". Expert Opin Investig Drugs. 14 (2): 177–84. doi:10.1517/13543784.14.2.177. PMID 15757393. S2CID 24694921.
- ↑ Elancheran, R.; Maruthanila, V. L.; Ramanathan, M.; Kabilan, S.; Devi, R.; Kunnumakara, A.; Kotoky, Jibon (2015). "Recent discoveries and developments of androgen receptor based therapy for prostate cancer". MedChemComm. 6 (5): 746–768. doi:10.1039/C4MD00416G. ISSN 2040-2503. S2CID 72654573.
- ↑ Leonard, Matthew J.; Lingham, Anthony R.; Niere, Julie O.; Jackson, Neale R. C.; McKay, Peter G.; Hügel, Helmut M. (2014). "Alternative synthesis of the anti-baldness compound RU58841". RSC Adv. 4 (27): 14143–14148. Bibcode:2014RSCAd...414143L. doi:10.1039/C4RA00332B. ISSN 2046-2069.
- 1 2 3 Cousty-Berlin D, Bergaud B, Bruyant MC, Battmann T, Branche C, Philibert D (October 1994). "Preliminary pharmacokinetics and metabolism of novel non-steroidal antiandrogens in the rat: relation of their systemic activity to the formation of a common metabolite". J. Steroid Biochem. Mol. Biol. 51 (1–2): 47–55. doi:10.1016/0960-0760(94)90114-7. PMID 7947350. S2CID 29752252.
Further reading
- Münster U, Nakamura C, Haberland A, Jores K, Mehnert W, Rummel S, Schaller M, Korting HC, Zouboulis CC, Blume-Peytavi U, Schäfer-Korting M (January 2005). "RU 58841-myristate--prodrug development for topical treatment of acne and androgenetic alopecia". Pharmazie. 60 (1): 8–12. PMID 15700772.