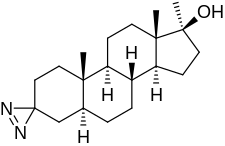
Methyldiazinol
 | |
| Clinical data | |
|---|---|
| Other names | Methyldiazirinol; 3,3-Azo-17α-methyl-5α-dihydrotestosterone; 3,3-Azo-17α-methyl-DHT; 3,3-Azo-17α-methyl-5α-androstan-17β-ol; 3-Azi-17α-methyl-5α-androstan-17β-ol |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H32N2O |
| Molar mass | 316.489 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Methyldiazinol (also known as 3,3-azo-17α-methyl-5α-dihydrotestosterone, 3-azi-17α-methyl-DHT, or 3,3-azo-17α-methyl-5α-androstan-17β-ol) is a synthetic and orally active androgen/anabolic steroid (AAS) which was never marketed.[1][2] It is a 17α-methylated derivative of dihydrotestosterone (DHT); specifically, it is the C3 azi (i.e., 3,3-azo) analogue of mestanolone (17α-methyl-DHT).[1][2] The drug has been found to possess a high ratio and dissociation of myotrophic to androgenic activity; relative to methyltestosterone, its ratio was 15 (3:0.2), among the highest observed.[1][2]
See also
References
- 1 2 3 Kochakian CD (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. p. 385. ISBN 978-3-642-66353-6.
Methyldiazinol: 3-Azi-17-methyl-5α-androstan-17β-ol: The 3-azi derivative of methandrostanolone was noted by CHURCH et al. (1965) to have a wide myotrophic:androgenic dissociation (3: 0.2) 15 relative to methyltestosterone orally, among the highest noted.
- 1 2 3 Church RF, Kende AS, Weiss MJ (1965). "Diazirines. I. Some Observations on the Scope of the Ammonia-Hydroxylamine-O-sulfonic Acid Diaziridine Synthesis. The Preparation of Certain Steroid Diaziridines and Diazirines". Journal of the American Chemical Society. 87 (12): 2665–2671. doi:10.1021/ja01090a025. ISSN 0002-7863.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.