Phenothrin
 | |
| Names | |
|---|---|
| IUPAC name
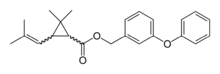
(3-Phenoxyphenyl)methyl 2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylate | |
| Other names
Sumithrin; Phenothrine; Phenoxythrin; Sumitrin; Wellcide; Pibutin; Anvil; Duet; Anchimanaito 20S | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
2160930 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.043.079 |
| EC Number |
|
| KEGG | |
| MeSH | Phenothrin |
PubChem CID |
|
| UNII | |
| UN number | 3082 2902 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C23H26O3 |
| Molar mass | 350.451 g/mol |
| Melting point | <25 °C |
| Boiling point | >290 °C |
| Pharmacology | |
| P03AC03 (WHO) QP53AC03 (WHO) | |
| Hazards[1] | |
| GHS labelling: | |
Pictograms |
  |
Signal word |
Warning |
Hazard statements |
H302, H312, H332, H410 |
Precautionary statements |
P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P322, P330, P363, P391, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phenothrin, also called sumithrin and d-phenothrin,[2] is a synthetic pyrethroid that kills adult fleas and ticks. It has also been used to kill head lice in humans. d-Phenothrin is used as a component of aerosol insecticides for domestic use. It is often used with methoprene, an insect growth regulator that interrupts the insect's biological lifecycle by killing the eggs.
Effects
Phenothrin is primarily used to kill fleas and ticks.[3] It is also used to kill head lice in humans, but studies conducted in Paris and the United Kingdom have shown widespread resistance to phenothrin.[3]
It is extremely toxic to bees. A U.S. Environmental Protection Agency (EPA) study found that 0.07 micrograms were enough to kill honey bees.[3] It is also extremely toxic to aquatic life with a study showing concentrations of 0.03 ppb killing mysid shrimp.[3] It has increased risk of liver cancer in rats and mice in long-term exposure.[3] It is capable of killing mosquitoes,[4] although remains poisonous to cats and dogs, with seizures and deaths being reported due to poisoning.[3] Specific data on concentrations or exposure are lacking.
Phenothrin has been found to possess antiandrogen properties, and was responsible for a small epidemic of gynecomastia via isolated environmental exposure.[5][6]
The EPA has not assessed its effect on cancer in humans. However, one study performed by the Mount Sinai School of Medicine linked sumithrin with breast cancer; the link made by its effect on increasing the expression of a gene responsible for mammary tissue proliferation.[3]
EPA action
In 2005, the U.S. EPA cancelled permission to use phenothrin in several flea and tick products, at the request of the manufacturer, Hartz Mountain Industries.[7][8] The products were linked to a range of adverse reactions, including hair loss, salivation, tremors, and numerous deaths in cats and kittens. In the short term, the agreement called for new warning labels on the products.
As of March 31, 2006, the sale and distribution of Hartz's phenothrin-containing flea and tick products for cats has been terminated. However, EPA's product cancellation order did not apply to Hartz flea and tick products for dogs, and Hartz continues to produce many of its flea and tick products for dogs.[9]
See also
References
- ↑ "Phenothrin". pubchem.ncbi.nlm.nih.gov.
- ↑ "d-Phenothrin". National Pesticide Information Center. Retrieved 2016-02-29.
- 1 2 3 4 5 6 7 Cox, Caroline (2003). "Insecticide Factsheet. Sumitherin (D-phenothrin)". Journal of Pesticide Reform. 23 (2): 10–14. Archived from the original on 2012-07-04. Retrieved 2012-08-16.
- ↑ "Permethrin, Resmethrin, d-Phenothrin (Sumithrin®): Synthetic Pyrethroids For Mosquito Control". US EPA. 21 February 2013.
- ↑ Barros, Alfredo Carlos Simões Dornellas de; Sampaio, Marcelo de Castro Moura (2012). "Gynecomastia: physiopathology, evaluation and treatment". Sao Paulo Medical Journal. 130 (3): 187–197. doi:10.1590/S1516-31802012000300009. ISSN 1516-3180. PMID 22790552.
Reinforcing the evidence suggesting that there is a relationship between chemicals and GM, it is worthwhile mentioning the epidemic onset observed among Haitian refugees in 1981 about four months after arrival in United States detention centers.22 After analyzing all identifiable environmental exposures, it was then found that phenothrin, a multi-insecticide contained in sprays that they had used was the causative agent.23 It is now widely known that phenothrin has antiandrogenic activity.
- ↑ Brody, Steven A.; Loriaux, D. Lynn (2003). "Epidemic of gynecomastia among haitian refugees: exposure to an environmental antiandrogen". Endocrine Practice. 9 (5): 370–5. doi:10.4158/EP.9.5.370. ISSN 1530-891X. PMID 14583418.
- ↑ Phenothrin and s-Methoprene; Product Cancellation Order, US Environmental Protection Agency
- ↑ Phenothrin; Amendment to Terminate Use, US Environmental Protection Agency
- ↑ "Archived copy". Archived from the original on 2009-06-06. Retrieved 2009-05-20.
{{cite web}}: CS1 maint: archived copy as title (link) See also "dog" in the following EPA ruling: "Insect Growth Regulators: S-Hydroprene (128966), S-Kinoprene (107502), Methoprene (105401), S-Methoprene (105402) Fact Sheet". U.S. Environmental Protection Agency. Archived from the original on 2009-06-24.
External links
- d-Phenothrin general information – National Pesticide Information Center
- Pyrethrins and Pyrethroids Fact Sheet – National Pesticide Information Center
- Pyrethrins and Pyrethroids Pesticide Information Profile – Extension Toxicology Network
- Phenothrin in the Pesticide Properties DataBase (PPDB)