Fluralaner
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌflʊərəˈlænər/ FLOOR-ə-LAN-ər |
| Trade names | Bravecto |
| Other names |
|
| License data |
|
| Routes of administration | By mouth (chewable tablets) |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 20–27%;[1] reduced in the fasted state[2] |
| Elimination half-life | 9.3–16.2 days[3] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.812 |
| Chemical and physical data | |
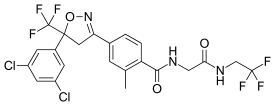
| Formula | C22H17Cl2F6N3O3 |
| Molar mass | 556.29 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Fluralaner (INN)[4] is a systemic insecticide and acaricide that is administered orally[5][6] or topically.[7] The U.S. Food and Drug Administration (FDA) approved it under the trade name Bravecto for flea treatment in dogs in May 2014[8] and Bravecto Plus as a topical treatment for cats in November 2019,[9] with warnings about possible side effects in both species.[10] The EU approved the drug in February 2014.[11] Australia approved it for the treatment and prevention of ticks and fleas on dogs in January 2015.[12]
Mode of action
Fluralaner inhibits γ-aminobutyric acid (GABA)-gated chloride channels (GABAA receptors) and L-glutamate-gated chloride channels (GluCls).[13] Potency of fluralaner is comparable to fipronil (a related GABA-antagonist insecticide and acaricide).[14]
See also
References
- 1 2 "Bravecto (fluralaner) for the Treatment and Prophylaxis of Arachnoenthomoses in Dogs. Full Prescribing Information" (PDF) (in Russian). Intervet GesmbH. Archived from the original (PDF) on 17 May 2018. Retrieved 14 November 2016.
- ↑ Walther FM, Allan MJ, Roepke RK, Nuernberger MC (March 2014). "The effect of food on the pharmacokinetics of oral fluralaner in dogs". Parasites & Vectors. 7 (1): 84. doi:10.1186/1756-3305-7-84. PMC 3975707. PMID 24598049.
- ↑ "Bravecto (fluralaner) Flavored Chews for Dogs. Prescribing Information" (PDF). Intervet, Inc., a subsidiary of Merck & Company, In. Retrieved 14 November 2016.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 69" (PDF). WHO Drug Information. 27 (1): 59. 2013. Archived from the original (PDF) on November 14, 2016. Retrieved 14 November 2016.
- ↑ Walther FM, Allan MJ, Roepke RK, Nuernberger MC (March 2014). "Safety of fluralaner chewable tablets (Bravecto), a novel systemic antiparasitic drug, in dogs after oral administration". Parasites & Vectors. 7 (1): 87. doi:10.1186/1756-3305-7-87. PMC 3975339. PMID 24606886.
- ↑ "CBD Dog Chews".
- ↑ Ranjan, Sivaja; Young, David; Sun, Fangshi (2018-07-03). "A single topical fluralaner application to cats and to dogs controls fleas for 12 weeks in a simulated home environment". Parasites & Vectors. 11 (1): 385. doi:10.1186/s13071-018-2927-0. PMC 6029119. PMID 29970135.
- ↑ Lee, J (21 May 2014). "New Flea/Tick Medication by Merck Just Approved: Bravecto". Retrieved 3 November 2014.
- ↑ "BRAVECTO® PLUS (fluralaner and moxidectin topical solution) for Cats Receives Approval from US Food and Drug Administration". Merck Animal Health. 2019-11-15. Retrieved 22 May 2021.
- ↑ Medicine, Center for Veterinary (2020-07-31). "Fact Sheet for Pet Owners and Veterinarians about Potential Adverse Events Associated with Isoxazoline Flea and Tick Products". FDA. Retrieved 22 May 2021.
- ↑ "MSD Animal Health receives EU approval for Bravecto". 19 February 2014. Retrieved 3 Nov 2014.
- ↑ "Agricultural and Veterinary Chemicals" (PDF). Australian Pesticides and Veterinary Medicines Authority. 10 February 2015. Retrieved 14 February 2019.
- ↑ Gassel M, Wolf C, Noack S, Williams H, Ilg T (February 2014). "The novel isoxazoline ectoparasiticide fluralaner: selective inhibition of arthropod γ-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity". Insect Biochemistry and Molecular Biology. 45: 111–24. doi:10.1016/j.ibmb.2013.11.009. PMID 24365472.
- ↑ Asahi M, Kobayashi M, Matsui H, Nakahira K (January 2015). "Differential mechanisms of action of the novel γ-aminobutyric acid receptor antagonist ectoparasiticides fluralaner (A1443) and fipronil". Pest Management Science. 71 (1): 91–5. doi:10.1002/ps.3768. PMID 24591229.