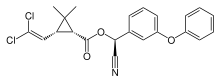
Cypermethrin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
[Cyano-(3-phenoxyphenyl)methyl]3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.052.567 |
| KEGG | |
| MeSH | Cypermethrin |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C22H19Cl2NO3 |
| Molar mass | 416.30 g/mol |
| Pharmacology | |
| P03BA02 (WHO) QP53AC08 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cypermethrin (CP) is a synthetic pyrethroid used as an insecticide in large-scale commercial agricultural applications as well as in consumer products for domestic purposes. It behaves as a fast-acting neurotoxin in insects. It is easily degraded on soil and plants but can be effective for weeks when applied to indoor inert surfaces. Exposure to sunlight, water and oxygen will accelerate its decomposition. Cypermethrin is highly toxic to fish, bees and aquatic insects, according to the National Pesticides Telecommunications Network (NPTN). It is found in many household ant and cockroach killers, including Raid, Ortho, Combat, and ant chalk.
Uses
Cypermethrin is used in agriculture to control ectoparasites which infest cattle, sheep, and poultry.[1]
Human exposure
Cypermethrin is moderately toxic through skin contact or ingestion. It may cause irritation to the skin and eyes. Symptoms of dermal exposure include numbness, tingling, itching, burning sensation, loss of bladder control, incoordination, seizures and possible death. Pyrethroids may adversely affect the central nervous system. Human volunteers given dermal doses of 130 μg/cm2 on the earlobe experienced local tingling and burning sensations. One man died after eating a meal cooked in a 10% cypermethrin/oil mix that was mistakenly used for cooking oil.[2] Shortly after the meal, the victim experienced nausea, prolonged vomiting, stomach pains, and diarrhea which progressed to convulsions, unconsciousness and coma. Other family members exhibited milder symptoms and survived after hospital treatment. It may cause allergic skin reactions in humans.[3] Excessive exposure can cause nausea, headache, muscle weakness, salivation, shortness of breath and seizures. In humans, cypermethrin is deactivated by enzymatic hydrolysis to several carboxylic acid metabolites, which are eliminated in the urine. Worker exposure to the chemical can be monitored by measurement of the urinary metabolites, while severe overdosage may be confirmed by quantitation of cypermethrin in blood or plasma.[4]
Study in animals
Cypermethrin is very toxic to cats which cannot tolerate the therapeutic doses for dogs.[5] This is associated with UGT1A6 deficiency in cats, the enzyme responsible for metabolizing cypermethrin. As a consequence, cypermethrin remains much longer in the cat's organs than in dogs or other mammals and can be fatal in large doses.
In male rats cypermethrin has been shown to exhibit a toxic effect on the reproductive system by Elbetieha et al 2001.[6] In another result, after 15 days of continual dosing, both androgen receptor levels and serum testosterone levels were significantly reduced. These data suggested that cypermethrin can induce impairments of the structure of seminiferous tubules and spermatogenesis in male rats at high doses.[7]
Long-term exposure to cypermethrin during adulthood is found to induce dopaminergic neurodegeneration in rats, and postnatal exposure enhances the susceptibility of animals to dopaminergic neurodegeneration if rechallenged during adulthood.[8]
If exposed to cypermethrin during pregnancy, rats give birth to offspring with developmental delays. In male rats exposed to cypermethrin, the proportion of abnormal sperm increases. It causes genetic damage: chromosomal abnormalities increased in bone marrow and spleen cells when mice were exposed to cypermethrin.[9] Cypermethrin is classified as a possible human carcinogen, because it causes an increase in the frequency of lung tumors in female mice. Cypermethrin has been linked to an increase in bone marrow micronuclei in both mice and humans.[10]
One study showed that cypermethrin inhibits “gap junctional intercellular communication”, which plays an important role in cell growth and is inhibited by carcinogenic agents.[11] Studies have shown that residue from cypermethrin can last for 84 days in the air, on walls, the floor and on furniture.[12]
Environmental effects
Cypermethrin is a broad-spectrum insecticide, which means it kills beneficial insects as well as the targeted insects.[13] Fish are particularly susceptible to cypermethrin,[14][15] but when used as directed, application around residential sites poses little risk to aquatic life.[16] Resistance to cypermethrin has developed quickly in insects exposed frequently and can render it ineffective.[17]
References
- ↑ "Cypermethrin". FAO.
- ↑ Ecobichon, Donald J. (1993). Pesticides and Neurological Diseases. CRC Press. p. 306. ISBN 978-0-8493-4361-2.
- ↑ "Cypermethrin". Extension Toxicology Network.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 389-391.
- ↑ Linnett, P-J (2008-01-01). "Permethrin toxicosis in cats". Australian Veterinary Journal. 86 (1–2): 32–35. doi:10.1111/j.1751-0813.2007.00198.x. ISSN 1751-0813. PMID 18271821.
- ↑ Koureas, Michalis; Tsakalof, Andreas; Tsatsakis, Aristidis; Hadjichristodoulou, Christos (2012). "Systematic review of biomonitoring studies to determine the association". Toxicology Letters. Elsevier. 210 (2): 155–168. doi:10.1016/j.toxlet.2011.10.007. ISSN 0378-4274.
- ↑ Hu, JX; Li, YF; Li, J; Pan, C; He, Z; Dong, HY; Xu, LC (2011). "Toxic effects of cypermethrin on the male reproductive system: With emphasis on the androgen receptor". Journal of Applied Toxicology. 33 (7): 576–585. doi:10.1002/jat.1769. PMID 22147539. S2CID 22178796.
- ↑ Singh, AK; Tiwari, MN; Upadhyay, G; Patel, DK; Singh, D; Prakash, O; Singh, MP (2012). "Long term exposure to cypermethrin induces nigrostriatal dopaminergic neurodegeneration in adult rats: Postnatal exposure enhances the susceptibility during adulthood". Neurobiology of Aging. 33 (2): 404–15. doi:10.1016/j.neurobiolaging.2010.02.018. PMID 20371137. S2CID 207158692.
- ↑ Amer, S.M.; et al. (1993). "Induction of chromosomal aberrations and sister chromatid exchange in vivo and in vitro by the insecticide cypermethrin". Journal of Applied Toxicology. 13 (5): 341–345. doi:10.1002/jat.2550130508. PMID 8258631. S2CID 41816737.
- ↑ Amer, S.M.; E.I. Aboulela (1985). "Cytogenetic effects of pesticides. III. Induction of micronuclei in mouse bone marrow by the insecticides cypermethrin and rotenone". Journal of Mutation Research. 155 (3): 135–142. doi:10.1016/0165-1218(85)90132-6. PMID 3974628.
- ↑ Tateno, C.; Ito, Seiichi; Tanaka, Mina; Yoshitake, Akira; et al. (1993). "Effects of pyrethroid insecticides on gap junctional intecellular communications in Balb/c3T3 cells by dye-transfer assay". Cell Biology and Toxicology Journal. 9 (3): 215–222. doi:10.1007/BF00755600. PMID 8299001. S2CID 10055706.
- ↑ Wright, C.G.; R.B. Leidy & H.E. Dupree Jr. (1993). "Cypermethrin in the ambient air and on surfaces of rooms treated for cockroaches". Bulletin of Environmental Contamination and Toxicology. 51 (3): 356–360. doi:10.1007/BF00201752. PMID 8219589. S2CID 37107396.
- ↑ Pascual, J.A.; S.J. Peris (1992). "Effects of forest spraying with two application rates of cypermethrin on food supply and on breeding success of the blue tit (Parus caeruleus)". Environmental Toxicology and Chemistry. 11 (9): 1271–1280. doi:10.1002/etc.5620110907.
- ↑ Stephenson, R.R. (1982). "Aquatic toxicology of cypermethrin. I. Acute toxicity to some freshwater fish and invertebrates in laboratory tests". Aquatic Toxicology. 2 (3): 175–185. doi:10.1016/0166-445X(82)90014-5.
- ↑ Ranjani, T. Sri; Pitchika, Gopi Krishna; Yedukondalu, K.; Gunavathi, Y.; Daveedu, T.; Sainath, S. B.; Philip, G. H.; Pradeepkiran, Jangampalli Adi (2020-02-07). "Phenotypic and transcriptomic changes in zebrafish (Danio rerio) embryos/larvae following cypermethrin exposure". Chemosphere. 249: 126148. Bibcode:2020Chmsp.249l6148R. doi:10.1016/j.chemosphere.2020.126148. ISSN 1879-1298. PMID 32062212. S2CID 211134591.
- ↑ "Cypermethrin" (PDF). National Pesticide Information Center.
- ↑ Martinez-Cabrillo, J.L.; et al. (1991). "Responses of populations of the tobacco budworm (Lepidopterea: Noctuidae) from northwest Mexico to pyrethroids". Journal of Economic Entomology. 84 (2): 363–366. doi:10.1093/jee/84.2.363.
External links
- Cypermethrin Fact Sheet - National Pesticide Information Center
- Cypermethrin Pesticide Information Profile - Extension Toxicology Network
- cypermethrin in the Pesticide Properties DataBase (PPDB)
- alpha-cypermethrin in the Pesticide Properties DataBase (PPDB)
- zeta-cypermethrin in the Pesticide Properties DataBase (PPDB)