Troleandomycin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a604026 |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.018.539 |
| Chemical and physical data | |
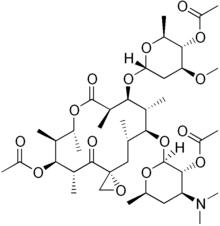
| Formula | C41H67NO15 |
| Molar mass | 813.979 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Troleandomycin (TAO for short[1]) is a macrolide antibiotic. It was sold in Italy (branded Triocetin) and Turkey (branded Tekmisin). It is no longer sold in Italy as of 2018.
The drug's mode of action is to bind to the ribosome, specifically in the tunnel through which the newly formed peptide egresses, thereby halting protein synthesis.[2] Troleandomycin is a CYP3A4 inhibitor that may cause drug interactions.
References
- ↑ Zeiger RS, Schatz M, Sperling W, Simon RA, Stevenson DD (December 1980). "Efficacy of troleandomycin in outpatients with severe, corticosteroid-dependent asthma". The Journal of Allergy and Clinical Immunology. 66 (6): 438–46. doi:10.1016/0091-6749(80)90003-2. PMID 6968762.
- ↑ Gürel G, Blaha G, Steitz TA, Moore PB (December 2009). "Structures of triacetyloleandomycin and mycalamide A bind to the large ribosomal subunit of Haloarcula marismortui". Antimicrobial Agents and Chemotherapy. 53 (12): 5010–4. doi:10.1128/AAC.00817-09. PMC 2786347. PMID 19738021.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.