Quinupristin/dalfopristin
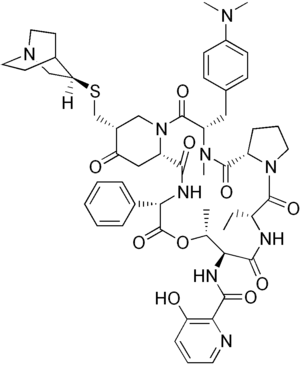 | |
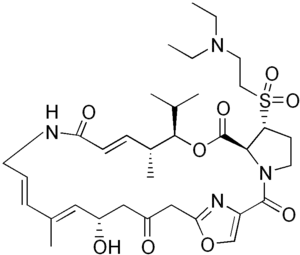 Quinupristin (top) and dalfopristin (bottom) | |
| Combination of | |
|---|---|
| Quinupristin | Streptogramin antibiotic |
| Dalfopristin | Streptogramin antibiotic |
| Names | |
| Pronunciation | kwi NYOO pris tin / dal FOE pris tin |
| Trade names | Synercid |
| Clinical data | |
| Drug class | Streptogramin antibiotics[1] |
| Main uses | Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE)[1] |
| Side effects | Pain at the site of injection, nausea, diarrhea, joint and muscle pain, headache, rash[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | IV |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
Quinupristin/dalfopristin, sold under the brand name Synercid, is a combination of two antibiotics used to treat infections by staphylococci, including Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE).[1] It is not very effective against VRE.[2] It is given by gradual injection into a vein.[1]
Common side effects include pain at the site of injection, nausea, diarrhea, joint and muscle pain, headache, and rash.[1] Other side effects may include Clostridioides difficile infection and anaphylaxis.[1] Quinupristin and dalfopristin are both streptogramin antibiotics, made from from pristinamycin.[1]
The combination was approved for medical use in the United States in 1999.[1] In the United States 10 doses of 350 mg/150 mg costs about 4,900 USD as of 2021.[3] In the United Kingdom it is no longer available as of 2018.[2]
Medical uses
Dosage
Intravenous, usually 7.5 mg/kg every 8 hours (infections/life threatening VRSA); every 12 hours (skin infections).[1]
Side effects
Common:
- Joint aches or muscle aches
- Nausea, diarrhea or vomiting
- Rash or itching
- Headache
- Hyperbilirubinemia
- Anemia
- Thrombophlebitis
Serious:
Interactions
The drug inhibits P450 and enhances the effects of terfenadine, astemizole, indinavir, midazolam, calcium channel blockers, warfarin, cisapride and ciclosporin.
Mechanism of action
Quinupristin and dalfopristin are protein synthesis inhibitors in a synergistic manner. While each of the two is only a bacteriostatic agent, the combination shows bactericidal activity.
- Dalfopristin binds to the 23S portion of the 50S ribosomal subunit, and changes the conformation of it, enhancing the binding of quinupristin[5] by a factor of about 100. In addition, it inhibits peptidyl transfer.[5]
- Quinupristin binds to a nearby site on the 50S ribosomal subunit and prevents elongation of the polypeptide,[5] as well as causing incomplete chains to be released.[5]
Pharmacokinetics
Clearance by the liver CYP450:3A4 inhibitor, half-life quinupristin 0.8 hours, dalfopristin 0.7 hours (with persistence of effects for 9–10 hours).
Excretion: Quinupristin: 85% feces, 15% urine; Dalfopristin: 81% feces, 19% urine
They are combined in a weight-to-weight ratio of 30% quinupristin to 70% dalfopristin.
Society and culture
Quinupristin is derived from pristinamycin IA; dalfopristin from pristinamycin IIA.[1]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Quinupristin/Dalfopristin Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 20 October 2021.
- 1 2 Barer, Michael R.; Irving, Will L. (13 January 2018). Medical Microbiology E-Book: A Guide to Microbial Infections. Elsevier Health Sciences. p. 53. ISBN 978-0-7020-7198-0. Archived from the original on 21 October 2021. Retrieved 20 October 2021.
- ↑ "Synercid Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 11 November 2020. Retrieved 20 October 2021.
- ↑ Epocrates v 19.5
- 1 2 3 4 Denyer SP, Hodges N, Gorman SP, eds. (2004). Hugo and Russell's Pharmaceutical microbiology (7th ed.). Blackwell Science. p. 212. ISBN 978-0-632-06467-0. Archived from the original on 2021-10-21. Retrieved 2021-10-16.
External links
- Allington DR, Rivey MP (January 2001). "Quinupristin/dalfopristin: a therapeutic review". Clinical Therapeutics. 23 (1): 24–44. doi:10.1016/S0149-2918(01)80028-X. PMID 11219478.
- Lamb HM, Figgitt DP, Faulds D (December 1999). "Quinupristin/dalfopristin: a review of its use in the management of serious gram-positive infections". Drugs. 58 (6): 1061–97. doi:10.2165/00003495-199958060-00008. PMID 10651391. S2CID 209144323.
- Manzella JP (December 2001). "Quinupristin-dalfopristin: a new antibiotic for severe gram-positive infections". American Family Physician. 64 (11): 1863–6. PMID 11764864..
- Paradisi F, Corti G, Messeri D (January 2001). "Antistaphylococcal (MSSA, MRSA, MSSE, MRSE) antibiotics" (PDF). The Medical Clinics of North America. 85 (1): 1–17. doi:10.1016/s0025-7125(05)70302-3. hdl:2158/329747. PMID 11190346. Archived (PDF) from the original on 2021-10-21. Retrieved 2021-10-16.
- "Synercid". U.S. Food and Drug Administration. Archived from the original on 2007-06-18.
| External sites: |
|
|---|---|
| Identifiers: |