Florfenicol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Nuflor |
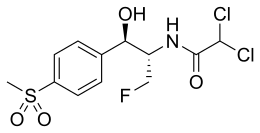
| Other names | 2,2-dichloro-N-((1R,2S)-3-fluoro-1-hydroxy-1-(4-(methylsulfonyl)phenyl)propan-2-yl)ethanamide |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | intramuscular, subcutaneous |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.170.898 |
| Chemical and physical data | |
| Formula | C12H14Cl2FNO4S |
| Molar mass | 358.21 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Florfenicol (marketed by Schering-Plough Animal Health under the trade name Nuflor) is a fluorinated synthetic analog of thiamphenicol,[1] mainly used in veterinary medicine.
Indications
In the United States, florfenicol is currently indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, for treatment of bovine interdigital phlegmon (foot rot, acute interdigital necrobacillosis, infectious pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus.
Florfenicol is also used in aquaculture, and is licensed for use in the United States for the control of enteric septicemia in catfish.[3]
Since the early 2000s, it is used in Europe,[4] treating mainly primary or secondary colibacillosis in broiler[5] and parent flocks. It is not allowed in laying hens, due to residues in eggs. It is also indicated in turkey.
The use of florfenicol in horses, and likely in other equids, typically causes diarrhea. This has been anecdotally reported to progress to lethal cases of acute colitis. Therefore, use of this antimicrobial in the equine patient should be limited to cases in which other, safer, options are not available.[6]
Contamination
Florfenicol was among the drug contaminants in a brand of supermarket eggs in Taiwan and Iran.[7]
External links
References
- ↑ Syriopoulou VP, Harding AL, Goldmann DA, Smith AL (February 1981). "In vitro antibacterial activity of fluorinated analogs of chloramphenicol and thiamphenicol". Antimicrob. Agents Chemother. 19 (2): 294–7. doi:10.1128/aac.19.2.294. PMC 181412. PMID 6957162.
- ↑ "Florfenicol".
- ↑ Gaunt, P. S.; Langston, C.; Wrzesinski, C.; Gao, D.; Adams, P.; Crouch, L.; Sweeney, D.; Endris, R. (2013). "Multidose pharmacokinetics of orally administered florfenicol in the channel catfish ( )". Journal of Veterinary Pharmacology and Therapeutics. 36 (5): 502–506. doi:10.1111/j.1365-2885.2012.01426.x. PMID 22882087.
- ↑ http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014277.pdf
- ↑ Shen, J.; Wu, X.; Jiang, H. (2002). "Pharmacokinetics of florfenicol in healthy and Escherichia coli-infected broiler chickens". Research in Veterinary Science. 73 (2): 137–140. doi:10.1016/s0034-5288(02)00033-4. PMID 12204631.
- ↑ Robinson, N.E.; Sprayberry, K.A. (2009). Current therapy in equine medicine. Saunders Elesevier. p. 13. ISBN 978-1-4160-5475-7. Retrieved March 21, 2011.
- ↑ Lee I-chia (8 January 2013). "Survey suggests certain eggs may be dangerous". Taipei Times. Retrieved 3 November 2014.