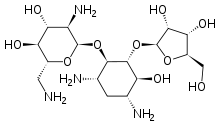
Ribostamycin
 | |
| Clinical data | |
|---|---|
| Other names | (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-{[(1R,2R,3S,4R,6S)-4,6-diamino-2-{[(2S,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-3-hydroxycyclohexyl]oxy}oxane-3,4-diol |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.053.421 |
| Chemical and physical data | |
| Formula | C17H34N4O10 |
| Molar mass | 454.477 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Ribostamycin is an aminoglycoside-aminocyclitol antibiotic isolated from a streptomycete, Streptomyces ribosidificus, originally identified in a soil sample from Tsu City of Mie Prefecture in Japan.[1] It is made up of 3 ring subunits: 2-deoxystreptamine (DOS), neosamine C, and ribose.[2] Ribostamycin, along with other aminoglycosides with the DOS subunit, is an important broad-spectrum antibiotic with important use against human immunodeficiency virus and is considered a critically important antimicrobial by the World Health Organization.,[3][4] Resistance against aminoglycoside antibiotics, such as ribostamycin, is a growing concern. The resistant bacteria contain enzymes that modify the structure through phosphorylation, adenylation, and acetylation and prevent the antibiotic from being able to interact with the bacterial ribosomal RNAs.[5]
Biosynthesis
The biosynthesis of ribostamycin begins with the sugar, D-glucose, which is phosphorylated at the 6 position to form glucose-6-phosphate. The enzyme rbmA contains a genetic sequence that corresponds to NAD+ binding and catalyzes the formation of 2-deoxy-scyllo-inosose. The enzyme rmbB then catalyzes the transamination of 2-deoxy-scyllo-inosose to 2-deoxy-scyllo-inosamine with L-glutamine and pyridoxal phosphate (PLP). Enzyme rbmC oxidizes the ring to 2-deoxy-3-amino-scyllo-inosose, which is then transaminated by enzyme rmbB to DOS. DOS is then glycosylated by the glycosyltransferase rmbD with uridine diphosphate N-acetylglucosamine (UDP-Glc-NAc) to form 2’-N-acetylparomamine. The deacetylase, racJ, removes the acetyl group and forms paromamine. Paromamine is oxidized by enzyme rbmG and then enzyme rmbH transaminates to produce neamine. Neamine is then ribosylated to form ribostamycin.,[2][3]
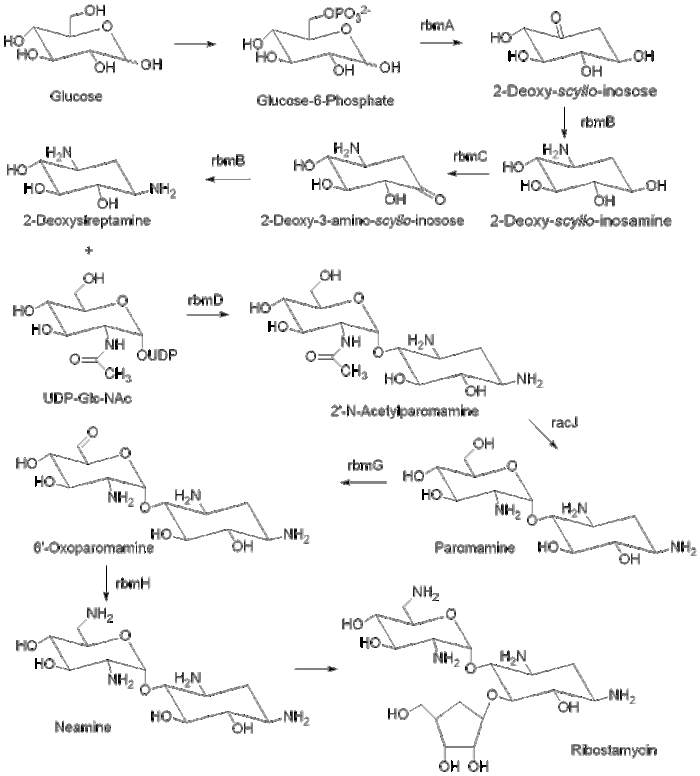 Biosynthetic patway for ribostamycin
Biosynthetic patway for ribostamycin
References
- ↑ Shomura T, Ezaki N, Tsuruoka T, Niwa T, Akita E (March 1970). "Studies on antibiotic SF-733, a new antibiotic. I. Taxonomy, isolation and characterization". The Journal of Antibiotics. 23 (3): 155–61. doi:10.7164/antibiotics.23.155. PMID 5453309.
- 1 2 Subba B, Kharel MK, Lee HC, Liou K, Kim BG, Sohng JK (August 2005). "The ribostamycin biosynthetic gene cluster in Streptomyces ribosidificus: comparison with butirosin biosynthesis". Molecules and Cells. 20 (1): 90–6. PMID 16258246.
- 1 2 Kurumbang NP, Liou K, Sohng JK (February 2011). "Biosynthesis of ribostamycin derivatives by reconstitution and heterologous expression of required gene sets". Applied Biochemistry and Biotechnology. 163 (3): 373–82. doi:10.1007/s12010-010-9045-6. PMID 20676801. S2CID 22366703.
- ↑ WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance (AGISAR) (2011). Critically Important Antimicrobials for Human Medicine (PDF) (Report) (3rd revision ed.). World Health Organization. ISBN 978-92-4-150448-5.
- ↑ Kudo F, Eguchi T (September 2009). "Biosynthetic genes for aminoglycoside antibiotics". The Journal of Antibiotics. 62 (9): 471–81. doi:10.1038/ja.2009.76. PMID 19644520. S2CID 41969498.