Totomycin
 | |
| Clinical data | |
|---|---|
| Other names | Hygromycin A, Homomycin |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C23H29NO12 |
| Molar mass | 511.480 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 110 to 112 °C (230 to 234 °F) (decomp.) |
SMILES
| |
InChI
| |
| | |
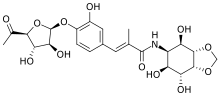
Hygromycin A (also known as totomycin) is a modified cinnamic acid flanked by a furanose sugar and aminocyclitol (not to be confused with hygromycin B, belonging to an unrelated class of antibiotics, aminoglycosides). It is produced by Streptomyces hygroscopicus, first described in the 1950s.
History
Hygromycin A was discovered in a soil sample from a forest near Indianapolis, Indiana in 1953 by Waksman and Henrici.[1] Identification and structure of totomycin was not determined until 1957.
Antibiotic activity
It was thought that the strongest antibiotic activity of totomycin was against Staphylococcus haemolyticus, in which growth was inhibited by concentrations of 2 µg/mL. Other gram-positive and gram-negative sensitive to totomycin are inhibited by concentrations from 16 to 31 µg/mL.[2]
In 2021 it was reported that Hygromycin A is very effective against spirochetes and can be used to eliminate the spirochete that causes Lyme disease. Bait laced with hygromycin A could be used to eliminate Lyme disease in the wild.[3]
Total synthesis
Totomycin has been a successful target in total synthesis since 1989.[4]
References
- ↑ Mann RL, Gale RM, Van Abelle FR (December 1953). "Hygromycin. II. Isolation and properties". Antibiotics & Chemotherapy. Northfield, Ill. 3 (12): 1279–82. PMID 24542809.
- ↑ GB 758276, "A new antibiotic Totomycin.", issued 1956, assigned to Jacques Loewe Research Foundation.
- ↑ Leimer N, Wu X, Imai Y, Morrissette M, Pitt N, Favre-Godal Q, et al. (October 2021). "A selective antibiotic for Lyme disease". Cell. 184 (21): 5405–5418.e16. doi:10.1016/j.cell.2021.09.011. PMC 8526400. PMID 34619078.
- ↑ Chida N, Ohtsuka M, Nakazawa K, Ogawa S (1989). "Total synthesis of hygromycin A". Journal of the Chemical Society, Chemical Communications. 7 (7): 436–8. doi:10.1039/C39890000436.