Omadacycline
 | |
| Names | |
|---|---|
| Pronunciation | oh mad" a sye' kleen |
| Trade names | Nuzyra |
| Other names | PTK-0796,[1] BAY 73-6944 |
| Clinical data | |
| Drug class | Antibiotic (tetracycline)[2] |
| Main uses | Community-acquired pneumonia, skin and skin structure infections[2] |
| Side effects | Liver problems, high blood pressure, trouble sleeping, diarrhea, headache[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618066 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
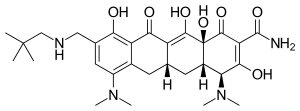
| Formula | C29H40N4O7 |
| Molar mass | 556.660 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Omadacycline, sold under the brand name Nuzyra, is an antibiotic used to treat community-acquired pneumonia and skin and skin structure infections.[2] It is taken by mouth or by injection into a vein.[2]
Common side effects include liver problems, high blood pressure, trouble sleeping, diarrhea, and headache.[2] Other side effects may include allergic reactions, tooth discoloration when used in those under 8 years old, and Clostridioides difficile infection.[2] It is a tetracycline antibiotics in the aminomethylcycline subclass.[2]
Omadacycline was approved for medical use in the United States in 2018.[2] It is not approved in either the United Kingdom or Europe as of 2021.[3] In the United States 14 days of treatment costs about 7,100 USD as of 2021.[4]
Medical use
In Europe it was declined approval for community required pneumonia due to insufficient evidence of benefit.[3]
Dosage
For pneumonia it is taken at a dose of 300 mg twice per day on the first day than 300 mg once per day for 7 to 14 days.[3]
Mechanism of action
The mechanism of action of omadacycline is similar to that of other tetracyclines – inhibition of bacterial protein synthesis. Omadacycline has activity against bacterial strains expressing the two main forms of tetracycline resistance (efflux and ribosomal protection).[5]
History
Omadacycline was invented at Tufts University School of Medicine by a research team led by Mark L. Nelson with Mohamed Ismail while at Tufts and Kwasi Ohemeng and Laura Honeyman at Paratek Pharmaceuticals, Boston. The team applying their chemistry methods to the tetracycline scaffolds created over 3000 new derivatives, leading to the novel third generation compounds omadacycline and sarecycline.[6]
Research
In vitro studies have shown that omadacycline has activity against a broad range of Gram-positive and select Gram-negative pathogens.[7] Omadacycline has potent in vitro activity against Gram-positive aerobic bacteria including methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant and multi-drug resistant Streptococcus pneumoniae, and vancomycin-resistant Enterococcus. Omadacycline also has antimicrobial activity against common Gram-negative aerobes, some anaerobes, and atypical bacteria such as Legionella and Chlamydia.[8] This activity translated to potent efficacy for omadacycline in an in vivo systemic infection model in mice.[9]
A phase II study was conducted comparing the safety and efficacy of omadacycline to linezolid for the treatment of complicated skin and skin structure infections. Patients were randomized at 11 sites in the US to receive either omadacycline 100 mg intravenously once daily with an option to transition to 200 mg orally once daily or linezolid 600 mg intravenously twice daily with an option to transition to 600 mg orally twice daily. The results indicated that omadacycline is well tolerated and has the potential to be an effective treatment in patients with complicated skin and skin structure infections.[10]
In June 2013, the US Food and Drug Administration (FDA) designated the intravenous and oral formulations of omadacycline as a qualified infectious disease product in the treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia.[11]
A 650 patient phase III registration study comparing omadacycline to linezolid for the treatment of acute bacterial skin and skin structure infections began in June 2015.[12][13] Omadacycline met the primary efficacy endpoint of early clinical response with statistical non-inferiority (10% margin) compared to linezolid, and was generally safe and well tolerated. The most common treatment-emergent adverse events were gastrointestinal side effects (18.0% for omadacycline vs. 15.8% for linezolid).[14]
A 750 patient phase III study comparing omadacycline to moxifloxacin for the treatment of community-acquired bacterial pneumonia began in November 2015.[15] Omadacycline was statistically non-inferior to moxifloxacin at the early clinical response, 72 to 120 hours after therapy was initiated.[16]
In May 2016, a phase Ib study of omadacycline in urinary tract infection was initiated.[17]
In August 2016, a second phase III study of omadacycline was initiated in patients with acute bacterial skin and skin structure infections, comparing the efficacy and safety of once-daily, oral omadacycline to that of twice-daily, oral linezolid.[18] In July 2017, analysis of the data showed that all of the primary and secondary endpoints required for submission to the FDA and EMA were met. This was the third phase 3 registration study of omadacycline with favorable results.[19]
References
- ↑ Boggs J. "Antibiotic Firm Paratek Joins IPO Queue; Aiming for $92M". bioworld.com. Clarivate Analytics. Archived from the original on October 18, 2017. Retrieved October 17, 2017.
- 1 2 3 4 5 6 7 8 9 "Omadacycline Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 7 November 2021.
- 1 2 3 "Omadacycline". SPS - Specialist Pharmacy Service. 1 January 2016. Archived from the original on 8 November 2021. Retrieved 7 November 2021.
- ↑ "Nuzyra Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 24 January 2021. Retrieved 7 November 2021.
- ↑ Draper MP, Weir S, Macone A, Donatelli J, Trieber CA, Tanaka SK, Levy SB (March 2014). "Mechanism of action of the novel aminomethylcycline antibiotic omadacycline". Antimicrobial Agents and Chemotherapy. 58 (3): 1279–83. doi:10.1128/AAC.01066-13. PMC 3957880. PMID 24041885.
- ↑ US 7056902, Nelson ML, Ohemeng K, "4-dedimethylamino tetracycline compounds", published 2006-06-06, assigned to Paratek Pharmaceuticals Inc.
- ↑ Tanaka SK, Villano S (September 2016). "In Vitro and In Vivo Assessments of Cardiovascular Effects with Omadacycline". Antimicrobial Agents and Chemotherapy. 60 (9): 5247–53. doi:10.1128/AAC.00320-16. PMC 4997885. PMID 27324778.
- ↑ Villano S, Steenbergen J, Loh E (October 2016). "Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections". Future Microbiology. 11 (11): 1421–1434. doi:10.2217/fmb-2016-0100. PMID 27539442.
- ↑ Macone AB, Caruso BK, Leahy RG, Donatelli J, Weir S, Draper MP, et al. (February 2014). "In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline". Antimicrobial Agents and Chemotherapy. 58 (2): 1127–35. doi:10.1128/AAC.01242-13. PMC 3910882. PMID 24295985.
- ↑ Noel GJ, Draper MP, Hait H, Tanaka SK, Arbeit RD (November 2012). "A randomized, evaluator-blind, phase 2 study comparing the safety and efficacy of omadacycline to those of linezolid for treatment of complicated skin and skin structure infections". Antimicrobial Agents and Chemotherapy. 56 (11): 5650–4. doi:10.1128/AAC.00948-12. PMC 3486554. PMID 22908151.
- ↑ "Paratek Pharmaceuticals Announces FDA Grant of Qualified Infectious Disease Product (QIDP) Designation for Its Lead Product Candidate, Omadacycline". prnewsire.com. PR Newswire. January 3, 2013. Archived from the original on October 18, 2017. Retrieved October 17, 2017.
- ↑ Seiffert D (2015). "Paratek presents new trial data for antibiotic as late-stage trials continue". bizjournals.com. American City Business Journals. Archived from the original on April 13, 2016. Retrieved October 17, 2017.
- ↑ Clinical trial number NCT02378480 for "Omadacycline Versus Linezolid for the Treatment of ABSSSI (EudraCT #2013-003644-23)" at ClinicalTrials.gov
- ↑ "Paratek Announces that Omadacycline Met All Primary and Secondary Efficacy Outcomes Designated by FDA and EMA in a Phase 3 Study in Acute Bacterial Skin Infections; Omadacycline was Generally Safe and Well-Tolerated". finance.yahoo.com. Archived from the original on 23 August 2016. Retrieved 3 July 2016.
- ↑ Clinical trial number NCT02531438 for "Omadacycline vs Moxifloxacin for the Treatment of CABP (EudraCT #2013-004071-13)" at ClinicalTrials.gov
- ↑ "Paratek Announces Positive Phase 3 Study of Omadacycline in Community-Acquired Bacterial Pneumonia". www.globenewswire.com. April 3, 2017. Archived from the original on 16 June 2017. Retrieved 16 May 2017.
- ↑ "Paratek Initiates Phase 1b Study of Omadacycline in Urinary Tract Infection". globenewswire.com. May 2, 2016. Archived from the original on 6 August 2016. Retrieved 3 July 2016.
- ↑ "Paratek Initiates Phase 3 Study of Oral-only Omadacycline in ABSSSI". globenewswire.com. August 15, 2016. Archived from the original on 15 August 2016. Retrieved 15 August 2016.
- ↑ "Paratek Announces Phase 3 Study of Oral-Only Dosing of Omadacycline Met All Primary and Secondary FDA and EMA Efficacy Endpoints in Acute Bacterial Skin Infections". www.globenewswire.com. July 17, 2017. Archived from the original on 20 July 2017. Retrieved 19 July 2017.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Omadacycline tosylate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-10-17. Retrieved 2021-10-06.
- "Omadacycline Injection". MedlinePlus. Archived from the original on 2021-08-18. Retrieved 2021-10-06.