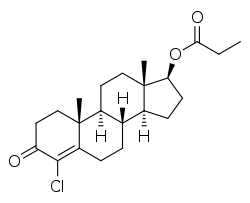
Clostebol propionate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Yonchlon |
| Routes of administration | Intramuscular injection |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H31ClO3 |
| Molar mass | 378.94 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Clostebol propionate (brand name Yonchlon), also known as 4-chlorotestosterone 17β-propionate or as 4-chloroandrost-4-en-17β-ol-3-one 17β-propionate, is a synthetic, injected anabolic-androgenic steroid (AAS) and a derivative of testosterone.[1][2] It is an androgen ester – specifically, the C17β propionate ester of clostebol (4-chlorotestosterone) – and acts as a prodrug of clostebol in the body.[1] Clostebol acetate is administered via intramuscular injection.[2]
See also
- Clostebol acetate
- Clostebol caproate
- Norclostebol
- Norclostebol acetate
- Oxabolone
- Oxabolone cipionate
References
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 305–. ISBN 978-1-4757-2085-3.
- 1 2 Morton IK, Hall JM (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 80–. ISBN 978-0-7514-0499-9.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.