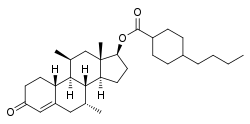
Dimethandrolone buciclate
 | |
| Clinical data | |
|---|---|
| Other names | CDB-4386A; 7α,11β-Dimethyl-19-nortestosterone 17β-buciclate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C31H48O3 |
| Molar mass | 468.722 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dimethandrolone buciclate (developmental code name CDB-4386A), or dimethandrolone bucyclate, also known as 7α,11β-dimethyl-19-nortestosterone 17β-buciclate, is a synthetic anabolic–androgenic steroid (AAS) and a derivative of nandrolone (19-nortestosterone) which was developed by the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development (NICHD) and has not been marketed at this time.[1][2][3][4][5] It is an androgen ester – specifically, the C17β buciclate (4-butylcyclohexane-1-carboxylate) ester of dimethandrolone (7α,11β-dimethyl-19-nortestosterone) – and acts as a prodrug of dimethandrolone in the body.[1][2][3][4][5] Dimethandrolone buciclate is or was under investigation as a potential male contraceptive.[1][2][3][4][5]
See also
References
- 1 2 3 US Abandoned 2003069215, Blye R, Kim H, "Methods of making and using 7a, 11b-dimethyl-17b-hydroxy-4-estren-3-one 17b-trans-4-n-butylcyclohexane carboxylate and 7a, 11b-dimethyl-17b-hydroxyestr-4-en-3-one 17-undecanoate.", published 10 April 2003, assigned to US Department of Health and Human Services
- 1 2 3 US Abandoned 2005130944, Blye R, Kim H, "Method of making and using 7alpha, 11beta-dimethyl-17beta-hydroxyestr-4-en-3-one 17-undecanoate.", published 16 June 2005, assigned to US Department of Health and Human Services
- 1 2 3 US Granted 7196074, Blye R, Kim H, "Methods of making, using and pharmaceutical formulations comprising 7α, 11β-dimethyl-17β-hydroxyestra-4, 14-dien-3-one and 17 esters thereof", issued 7 March 2007, assigned to US Department of Health and Human Services
- 1 2 3 US Abandoned 2009023695, Blye R, Kim H, "Method of making and using 7alpha, 11beta-dimethyl-17beta-hydroxyestr-4-en-3-one 17-undecanoate.", published 22 January 2009, assigned to US Department of Health and Human Services
- 1 2 3 Brown AE, Sorbera LA (2013). "Therapeutic targets for male contraception". Drugs of the Future. 38 (7): 499. doi:10.1358/dof.2013.038.07.1980494. ISSN 0377-8282.