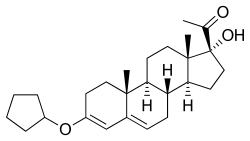
Pentagestrone
 | |
| Clinical data | |
|---|---|
| Other names | 17α-Hydroxyprogesterone 3-cyclopentyl enol ether |
| Drug class | Progestogen ether |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H38O3 |
| Molar mass | 398.587 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pentagestrone (INN), also known as 17α-hydroxyprogesterone 3-cyclopentyl enol ether, is a steroidal progestin of the 17α-hydroxyprogesterone group that was never marketed.[1][2] An acetate ester, pentagestrone acetate (Gestovis, Gestovister), has been marketed for clinical use.[1] Pentagestrone was described in the literature in 1960.[1]
See also
References
- 1 2 3 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 943–. ISBN 978-1-4757-2085-3.
- ↑ Wermuth CG (2 May 2011). The Practice of Medicinal Chemistry. Academic Press. pp. 731–. ISBN 978-0-08-056877-5.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.