Norgesterone
 | |
| Clinical data | |
|---|---|
| Trade names | Vestalin (with EE) |
| Other names | Norvinodrel; Vinylestrenolone; Vinilestrenolone; Vinylnoretynodrel; 17α-Vinylestr-5(10)-en-17-ol-3-one; 17α-Vinyl-δ5(10)-19-nortestosterone |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Norgesterone, also known as norvinodrel or vinylestrenolone and sold under the brand name Vestalin, is a progestin medication which was formerly used in birth control pills for women but is now no longer marketed.[1][2][3][4] It was used in combination with the estrogen ethinylestradiol.[2][3][4] It is taken by mouth.[5][6]
Norgesterone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[7] It has no androgenic activity.[7]
Norgesterone was first described in 1962.[8][9] It is no longer available.[10]
Medical uses
Norgesterone was used in combination with ethinylestradiol in birth control pills to prevent pregnancy.[2] It is no longer available.[10]
Pharmacology
Pharmacodynamics
Norgesterone is a progestogen, and hence is an agonist of the progesterone receptor.[7] Unlike related progestins, it is virtually devoid of androgenic activity in animal assays.[7]
Chemistry
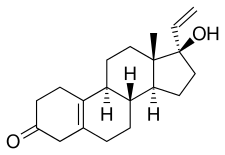
Norgesterone, also known as 17α-vinyl-δ5(10)-19-nortestosterone or as 17α-vinylestr-5(10)-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone and 19-nortestosterone.[1] Analogues of norgesterone include norvinisterone (17α-vinyl-19-nortestosterone) and vinyltestosterone (17α-vinyltestosterone).[1]
History
Society and culture
Generic names
Norgesterone is the generic name of the drug and its INN.[1] It has also been referred to as norvinodrel, vinylestrenolone, and vinylnoretynodrel.[1][11]
Brand names
Norgesterone was marketed in combination with ethinylestradiol, an estrogen, as a birth control pill under the brand name Vestalin.[2][3][4]
Availability
Norgesterone is no longer marketed and hence is no longer available in any country.[10]
References
- 1 2 3 4 5 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 Wassef SA, Sami G, Hamid EA (1970). "Effect of switching with oral contraceptives". Egypt Popul Fam Plann Rev. 3 (1): 77–93. PMID 12254511.
- 1 2 3 Lars Philip Bengtsson; M. Tausk (September 1972). Pharmacology of the endocrine system and related drugs: progesterone, progestational drugs and antifertility agents. Pergamon Press. ISBN 9780080157450.
- 1 2 3 Cynthia A. Challener (1 December 2001). Chiral Drugs. Wiley. ISBN 978-0-566-08411-9.
- ↑ Boris Rubio L (November 1966). "[Vinylestrenolone: a new progestational hormone. Results of its cyclic administration]". Minerva Ginecol (in Italian). 18 (21): 1215–7. PMID 5997085.
- ↑ Samaja, B. A., & Prandini, B. (1974). The influence of estrogenic and/or progestogenic treatment on some parameters of lipid metabolism: a controlled clinical study. Endokrinologie, 63(1), 76-84. https://www.popline.org/node/492815
- 1 2 3 4 Ruggieri, Pietro de; Matscher, Rodolfo; Lupo, Corrado; Spazzoli, Giacomo (1965). "Biological properties of 17α-vinyl-5(10)-estrene-17β-ol-3-one (norvinodrel) as a progestational and claudogenic compound". Steroids. 5 (1): 73–91. doi:10.1016/0039-128X(65)90133-9. ISSN 0039-128X.
- 1 2 "Steroid hormone compositions and method of using same".
- 1 2 D'Incerti Bonini L, Pagani C (April 1962). "[Clinical investigations of the progestational activity of vinylestrenolone]". Ann Ostet Ginecol (in Italian). 84: 279–85. PMID 13883015.
- 1 2 3 http://www.micromedexsolutions.com/micromedex2/%5B%5D
- ↑ J.P. Lavery; J.S. Sanfilippo (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 236–. ISBN 978-1-4612-5064-7.