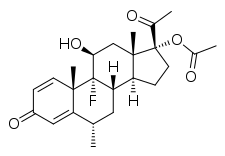
Fluorometholone acetate
 | |
| Names | |
|---|---|
| Trade names | Flarex, Florate, Omnitrol, others |
| Other names | Oxylone acetate; 1-Dehydro-9α-fluoro-11β,17α-dihydroxy-6α-methylprogesterone 17α-acetate; 17α-Acetoxy-9α-fluoro-11β-hydroxy-6α-methylpregna-1,4-diene-3,20-dione |
IUPAC name
| |
| Clinical data | |
| Drug class | Corticosteroid; Glucocorticoid; Progestogen |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Chemical and physical data | |
| Formula | C24H31FO5 |
| Molar mass | 418.505 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Fluorometholone acetate, also known as oxylone acetate and sold under the brand names Flarex among others, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester, as well as a progestogen and progestogen ester.[1][2][3][4]: 186 It is the C17α acetate ester of fluorometholone.[1][2][3]
In addition to its potent corticosteroid activity, fluorometholone acetate is a progestogen.[4] It has been studied in the treatment of breast cancer in women and has been found to be effective, producing remission in about 20% of women with advanced breast cancer at an oral dosage of 50 mg/day.[4] However, it also produces severe Cushing's syndrome-like symptoms like plethora, moon face, glycosuria, marked weight gain, hypertension, and osteoporosis at this dosage due to its glucocorticoid activity.[4]
See also
References
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 566–. ISBN 978-1-4757-2085-3. Archived from the original on 1 August 2020. Retrieved 21 November 2020.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 458–459. ISBN 978-3-88763-075-1. Archived from the original on 2020-08-01. Retrieved 2020-11-21.
- 1 2 Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 123–. ISBN 978-94-011-4439-1. Archived from the original on 1 August 2020. Retrieved 21 November 2020.
- 1 2 3 4 Dao TL (1975). "Pharmacology and Clinical Utility of Hormones in Hormone Related Neoplasms". In Sartorelli AC, Johns DG (eds.). Antineoplastic and Immunosuppressive Agents. pp. 170–192. doi:10.1007/978-3-642-65806-8_11. ISBN 978-3-642-65806-8. Archived from the original on 2021-02-15. Retrieved 2020-11-21.
Oxylone acetate (progesterone, 1-dehydro-9-fluoro-11β,17-dihydroxy-6α-methyl-17-acetate, fluorometholone), another halogenated progesterone, has been studied in patients with breast cancer (Cooperative Breast Cancer Group, 1964). This compound, given at a dose level of 50 mg per day orally, was shown to produce remission in about 20 % of the patients with advanced breast cancer. Both of these progestational compounds possess corticoid activity, particularly oxylone acetate, which, in 50 mg doses, causes Cushingoid symptoms, hypertension, and osteoporosis. On withdrawal of the drug, vaginal bleeding occurs frequently. [...] Progesterone-like compounds with glucocorticoid activity, such as oxylone, induce severe Cushingoid symptoms such as plethora, moonface, glycosuria, marked weight gain, and hypertension, requiring discontinuation of the drug.
External links
| Identifiers: |
|---|