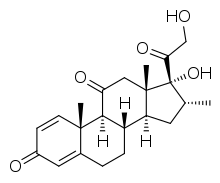
16α-Methyl-11-oxoprednisolone
 | |
| Clinical data | |
|---|---|
| Other names | Dexamethasone impurity J; 17α,21-Dihydroxy-16α-methylpregna-1,4-diene-3,11,20-trione |
| Drug class | Corticosteroid; Glucocorticoid |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H28O5 |
| Molar mass | 372.461 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
16α-Methyl-11-oxoprednisolone, also known as dexamethasone impurity J, is a synthetic glucocorticoid corticosteroid which was reported in 1979 and was never marketed.[1][2]
References
- ↑ Lutsky BN, Berkenkopf J, Fernandez X, Monahan M, Shue HJ, Tiberi RL, Green MJ (1979). "A novel class of potent topical antiinflammatory agents: 17-benzoylated, 7 alpha-halogeno substituted corticosteroids". Arzneimittelforschung. 29 (11): 1662–7. PMID 543873.
- ↑ "Custom Chemical Quote - Toronto Research Chemicals | Products for innovative research".
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.