Benidipine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
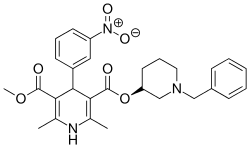
| Formula | C28H31N3O6 |
| Molar mass | 505.571 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Benidipine is a dihydropyridine calcium channel blocker for the treatment of high blood pressure (hypertension). It is a triple L-, T-, and N-type calcium channel blocker. It is reno- and cardioprotective.
It was patented in 1981 and approved for medical use in 1991.[1]
Dosing
Benidipine is dosed as 2–8 mg once daily.[2]
Mechanism
Benidipine is a calcium channel blocker.
Benidipine has additionally been found to act as an antagonist of the mineralocorticoid receptor, or as an antimineralocorticoid.[3]
Names
Other names include Benidipinum or benidipine hydrochloride.
Benidipine is sold as Coniel by Kyowa Hakko Kogyo.
Benidipine is initially licensed for use in Japan and selected Southeast Asian countries and later in Turkey, where it is sold as 4 mg tablets.
References
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 465. ISBN 9783527607495.
- ↑ Hi-Eisai Pharmaceutical, Inc. "Coniel (benidipine) package insert (Philippines)". MIMS Philippines. CMPMedica. Retrieved 2008-03-31.
- ↑ Luther JM (September 2014). "Is there a new dawn for selective mineralocorticoid receptor antagonism?". Current Opinion in Nephrology and Hypertension. 23 (5): 456–61. doi:10.1097/MNH.0000000000000051. PMC 4248353. PMID 24992570.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.