Propafenone
 | |
 | |
| Names | |
|---|---|
| Pronunciation | /proʊˈpæfɪnoʊn/ proh-PAF-i-nohn |
| Trade names | Rythmol, Rytmonorm, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Class 1c anti-arrhythmic medication[1] |
| Main uses | Supraventricular and ventricular tachyarrhythmias[1] |
| Side effects | Nausea, dizziness, headache, blurry vision, shortness of breath, liver problems[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698002 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 97% |
| Elimination half-life | 2–10 hours |
| Chemical and physical data | |
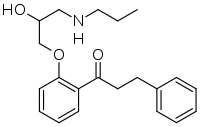
| Formula | C21H27NO3 |
| Molar mass | 341.451 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Propafenone, sold under the brand name Rythmol among others, is a medication used to treat supraventricular and ventricular tachyarrhythmias.[1] Long term use is generally not recommended.[3] It is taken by mouth.[1]
Common side effects include nausea, dizziness, headache, blurry vision, shortness of breath, and liver problems.[1] Other side effects may include heart failure, other arrhythmias, and low white blood cells.[1] Use is not recommended in people with structural heart disease or ischemic heart disease.[1] It is a class 1c anti-arrhythmic medication.[1]
Propafenone was approved for medical use in the United States in 1989.[1] It is available as a generic medication.[4] In the United Kingdom a typical dose costs the NHS about £7 a month.[4] In the United States this amount is 15 USD per month.[5] A long acting formulation is also available.[1]
Medical uses
Supraventricular
It may be used for atrial flutter, atrial fibrillation, and atrioventricular reentrant tachycardias.[4]
In atrial fibrillation a single dose of 600 mg maybe used to try to convert the arrhythmia with success rates around 40%.[6] This has been used by outpatients on an occasional basis, after verification in hospital of no adverse effects.[6]
Ventricular
Use is generally only recommended in people with ventricular arrhythmias that are life threatening.[1]
Dosage
Propafenone is generally started at 150 mg three times per day; though may be increased to 300 mg twice or three times per day.[4] The maximal dose is 900 mg/d.[4]
For economic and convenience reasons, some clinicians are starting certain antiarrhythmic agents in an outpatient setting. No consensus exists regarding the safety of this practice, and information is needed to determine which agents and which patients are appropriate for outpatient initiation of antiarrhythmic therapy. From a clinical point of view, this drug is used primarily in patients with relatively preserved myocardial function.[7]
Side effects
Side effects attributed to propafenone include hypersensitivity reactions, lupus-like syndrome, agranulocytosis, CNS disturbances such as dizziness, lightheadedness, gastrointestinal upset, a metallic taste and bronchospasm. About 20% of people discontinued the drug due to side effects.
Caution should be used in administrating propafenone in individuals with hepatic dysfunction, asthma, congestive heart failure, or bradycardia.
Mechanism of action
Propafenone works by slowing the influx of sodium ions into the cardiac muscle cells, causing a decrease in excitability of the cells. Propafenone is more selective for cells with a high rate, but also blocks normal cells more than class Ia or Ib anti-arrhythmic medications. Propafenone differs from the prototypical class Ic antiarrhythmic in that it has additional activity as a beta-adrenergic blocker which can cause bradycardia and bronchospasm.
Metabolism
Propafenone is metabolized primarily in the liver. Because of its short half-life, it requires dosing two or three times daily to maintain steady blood levels. The long-term safety of propafenone is unknown. Because it is structurally similar to another anti-arrhythmic medicine, flecainide, similar cautions should be exercised in its use. Flecainide and propafenone, like other antiarrhythmic drugs have been shown to increase the occurrence of arrhythmias (5.3% for propafenone, Teva physician prescribing information), primarily in patients with underlying heart disease. However, their use in structurally normal hearts is considered safe.
Chemistry
Propafenone contains a stereocenter and consists of two enantiomers. This is a racemate, a 1:1 mixture of (R)– and (S)–forms:[8]
| Enantiomers of propafenone | |
|---|---|
-Propafenon_Structural_Formula_V1.svg.png.webp) (R)-propafenone CAS number: 107381-31-7 |
-Propafenon_Structural_Formula_V1.svg.png.webp) (S)-propafenone CAS number: 107381-32-8 |
History
Propafenone was approved for use in the United States in November 1989.[9][10]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Propafenone Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 29 October 2021.
- ↑ "Propafenone Use During Pregnancy". Drugs.com. 13 November 2018. Archived from the original on 7 February 2020. Retrieved 7 February 2020.
- ↑ Bersten, Andrew D.; Handy, Jonathan (15 August 2018). Oh's Intensive Care Manual E-Book. Elsevier Health Sciences. p. 271. ISBN 978-0-7020-7606-0. Archived from the original on 30 October 2021. Retrieved 29 October 2021.
- 1 2 3 4 5 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 112. ISBN 978-0857114105.
- ↑ "Propafenone Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 29 October 2021.
- 1 2 Deneer, VH; Borgh, MB; Kingma, JH; Lie-A-Huen, L; Brouwers, JR (April 2004). "Oral antiarrhythmic drugs in converting recent onset atrial fibrillation". Pharmacy world & science : PWS. 26 (2): 66–78. doi:10.1023/b:phar.0000018593.02291.c0. PMID 15085940.
- ↑ "Clinical Guidelines and Recommendations". www.ahrq.gov. Archived from the original on 7 February 2012. Retrieved 23 August 2019.
- ↑ F. v. Bruchhausen, G. Dannhardt, S. Ebel, A. W. Frahm, E. Hackenthal, U. Holzgrabe (Hrsg.): Hagers Handbuch der Pharmazeutischen Praxis: Band 9: Stoffe P–Z, Springer Verlag, Berlin, Aufl. 5, 2014, S. 387, ISBN 978-3-642-63389-8.
- ↑ "Drugs@FDA: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 19 October 2020. Retrieved 6 February 2020.
- ↑ "Drug Approval Package: Rythmol SR (Propafenone Hydrochloride) NDA #021416". U.S. Food and Drug Administration (FDA). 5 May 2004. Archived from the original on 7 February 2020. Retrieved 6 February 2020.
External links
- Dean L (2017). "Propafenone Therapy and CYP2D6 Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520383. Bookshelf ID: NBK425391. Archived from the original on 26 October 2020. Retrieved 22 September 2021.
| External sites: |
|
|---|---|
| Identifiers: |