Esmolol
 | |
| Names | |
|---|---|
IUPAC name
| |
| Clinical data | |
| Drug class | Cardioselective beta1 receptor blocker |
| Main uses | Supraventricular arrhythmias, high blood pressure, myocardial ischemia[1] |
| Side effects | Low blood pressure, sweating, headache, nausea, inflammation at the site of injection[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous[1] |
| Onset of action | Within a min[1] |
| Duration of action | Up to 30 min[1] |
| Typical dose | 50 to 200 ug/kg/min[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Pharmacokinetics | |
| Bioavailability | - |
| Protein binding | 60% |
| Metabolism | Erythrocytic |
| Elimination half-life | 9 minutes[3] |
| Excretion | Kidney |
| Chemical and physical data | |
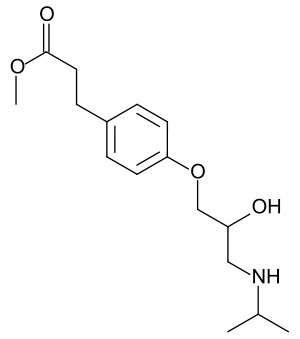
| Formula | C16H25NO4 |
| Molar mass | 295.379 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Esmolol, sold under the brand name Brevibloc among others, is a medication used to treat supraventricular arrhythmias, high blood pressure, and myocardial ischemia.[1] It is given by injection into a vein.[1] Onset of effects can begin within a minute and last for up to 30 minutes.[1]
Common side effects include low blood pressure, sweating, headache, nausea, and inflammation at the site of injection.[1] Other side effects may include swelling, pulmonary edema, and urinary retention.[2] It is a cardioselective beta1 receptor blocker which inhibits the sympathetic nervous system.[4][5] It is a class II antiarrhythmic.[6]
Esmolol was patented in 1980 and approved for medical use in 1986.[7][1] It is available as a generic medication.[2] In the United Kingdom it costs the NHS about £10 per 100 mg vial.[2] In the United States this amount is about 6 USD as of 2021.[8]
Medical uses
To treat supraventricular tachycardia including atrial fibrillation or atrial flutter.
Arrhythmia during anaesthesia,
To reduce HR and BP during and after cardiac surgery.
In the early treatment of myocardial infarction.
Esmolol is also used in blunting the hemodynamic response to laryngoscopy and intubation.[9]
Dosing
It is generally given at a dose of 50 to 200 ug/kg/min.[2]
Metabolism
Esmolol is considered a soft drug,[10] one that is rapidly metabolized to an inactive form. Esmolol is rapidly metabolized by hydrolysis of the ester linkage, chiefly by the esterases in the cytosol of red blood cells and not by plasma cholinesterases or red cell membrane acetylcholinesterase. Total body clearance in man was found to be about 20 L/kg/hr, which is greater than cardiac output; thus the metabolism of esmolol is not limited by the rate of blood flow to metabolizing tissues such as the liver or affected by hepatic or renal blood flow. Esmolol's short duration of action is based on the ester-methyl side chain which allows for quick hydrolysis. Esmolol's structure is reflected in its name, es-molol as in ester-methyl. Plasma cholinesterases and red cell membrane acetylcholinesterase do not have any action. This metabolism results in the formation of a free acid and methanol. The amount of methanol produced is similar to endogenous methanol production. Esmolol has a rapid distribution half-life of about two minutes.
It has a half-life of about nine minutes.[3]
References
- 1 2 3 4 5 6 7 8 9 10 "Esmolol Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 23 July 2021.
- 1 2 3 4 5 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 166. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 Benowitz, Neal L. (2020). "11. Antihypertensive agents". In Katzung, Bertram G.; Trevor, Anthony J. (eds.). Basic and Clinical Pharmacology (15th ed.). New York: McGraw-Hill. p. 193. ISBN 978-1-260-45231-0. Archived from the original on 2021-10-10. Retrieved 2021-12-05.
- ↑ Barash, Paul G.; Cullen, Bruce F.; Stoelting, Robert K.; Cahalan, Michael; Stock, M. Christine (1 January 2011). Clinical Anesthesia. Lippincott Williams & Wilkins. p. PT1598. ISBN 978-1-4511-2297-8. Archived from the original on 28 August 2021. Retrieved 23 July 2021.
- ↑ Sharar, Sam R.; Cullen, Bruce F.; Stock, Christine M.; Ortega, Rafael; Holt, Natalie; Nathan, Naveen; Connor, Christopher (9 July 2021). Clinical Anesthesia Fundamentals. Lippincott Williams & Wilkins. p. PT335. ISBN 978-1-9751-1302-5. Archived from the original on 28 August 2021. Retrieved 23 July 2021.
- ↑ Marx, John; Hockberger, Robert; Walls, Ron (1 August 2013). Rosen's Emergency Medicine - Concepts and Clinical Practice E-Book: 2-Volume Set. Elsevier Health Sciences. p. 1038. ISBN 978-1-4557-4987-4. Archived from the original on 28 August 2021. Retrieved 23 July 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 462. ISBN 978-3-527-60749-5. Archived from the original on 2021-06-10. Retrieved 2021-03-18.
- ↑ "Esmolol Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 28 August 2021. Retrieved 23 July 2021.
- ↑ Sharma S, Suthar OP, Tak ML, Thanvi A, Paliwal N, Karnawat R (2018). "Comparison of Esmolol and Dexmedetomidine for Suppression of Hemodynamic Response to Laryngoscopy and Endotracheal Intubation in Adult Patients Undergoing Elective General Surgery: A Prospective, Randomized Controlled Double-blinded Study". Anesthesia: Essays and Researches. 12 (1): 262–266. doi:10.4103/aer.AER_226_17. PMC 5872877. PMID 29628593.
- ↑ Bodor N, Buchwald P (2000). "Soft drug design: General principles and recent applications". Medicinal Research Reviews. 20 (1): 58–101. doi:10.1002/(SICI)1098-1128(200001)20:1<58::AID-MED3>3.0.CO;2-X. PMID 10608921.
External links
| External sites: |
|
|---|---|
| Identifiers: |