Nadolol
 | |
| Names | |
|---|---|
| Trade names | Corgard, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Beta blocker (non-selective) |
| Main uses | Treat: high blood pressure, heart pain, atrial fibrillation[1] Prevent: Migraines, complications of cirrhosis[2][3] |
| Side effects | Dizziness, feeling tired, slow heart rate, Raynaud syndrome[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Defined daily dose | 160 mg[4] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682666 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 30% |
| Metabolism | Not metabolised |
| Elimination half-life | 14-24 hours |
| Excretion | Kidney and fecal (unchanged) |
| Chemical and physical data | |
| Formula | C17H27NO4 |
| Molar mass | 309.406 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Nadolol, sold under the brand name Corgard among others, is a medication used to treat high blood pressure, heart pain, and atrial fibrillation.[1] It has also been used to prevent migraine headaches and complications of cirrhosis.[2][3] It is taken by mouth.[2]
Common side effects include dizziness, feeling tired, a slow heart rate, and Raynaud syndrome.[1] Serious side effects may include heart failure and bronchospasm.[1] Its use in pregnancy and breastfeeding is of unclear safety.[5] It is a non-selective beta blocker and works by blocking β1-adrenergic receptors in the heart and β2-adrenergic receptors in blood vessels.[1]
Nadolol was patented in 1970 and came into medical use in 1978.[6] It is available as a generic medication.[1] A month supply in the United Kingdom costs the NHS about GB£6 as of 2019.[2] In the United States the wholesale cost of this amount is about US$52 .[7] In 2016, it was the 283rd most commonly prescribed medication in the United States with more than a million prescriptions.[8]
Medical uses
Nadolol is used to treat high blood pressure and for long-term treatment of angina.[9]
Other uses include for the control of heart rate in people with atrial fibrillation,[10] prevention of migraines;[11] prevention of bleeding veins in people with portal hypertension caused by cirrhosis;[3] and to treat people with high levels of thyroid hormone.[12]
Nadolol is one of the preferred beta-blockers in the management of people with LQTS for shortening of the QT interval and prevention of ventricular arrhythmia. It is more efficacious than cardioselective beta-blockers like metoprolol and propanolol in the prevention of breakthrough cardiac events.[13] Nadolol has the advantage of once daily dosing and thus improved patient compliance. For patients with decreased kidney function, nadolol may be dosed less often.[14] It has also been found to be useful for the prevention of migraine attacks,[15] attention deficit/hyperactivity disorder(ADHD)[16] and its use has been explored as a treatment for essential tremor[17] and Parkinson's disease[18] but neither is well established.[19][20][21]
Dosage
The defined daily dose is 160 mg by mouth.[4]
Side effects
The most common side effects include dizziness and fatigue.[18]
Nadolol and other beta blockers should be used with cautions in people with heart failure and its use should not be abruptly stopped. It is contraindicated for people with asthma, a slow heart rate and certain severe heart problems.[22]
Mechanism of action
Nadolol is a non-selective beta blocker; that is, it non-selectively blocks both beta-1 and beta-2 receptors. It has a preference for beta-1 receptors, which are predominantly located in the heart, thereby inhibiting the effects of catecholamines and causing a decrease in heart rate and blood pressure. Its inhibition of beta-2 receptors, which are mainly located in the bronchial smooth muscle of the airways, leads to airway constriction similar to that seen in asthma. Inhibition of beta-1 receptors in the juxtaglomerular apparatus of the kidney inhibits the renin–angiotensin system, causing a decrease in vasoconstriction and a decrease in water retention. Nadolol's inhibition of beta-1 receptors in the heart and kidney leads to its effects on lowering blood pressure.
The drug impairs AV node conduction and decreases sinus rate.
Nadolol may also increase plasma triglycerides and decrease HDL-cholesterol levels.
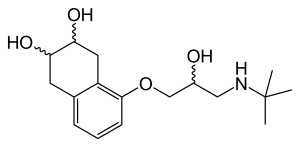
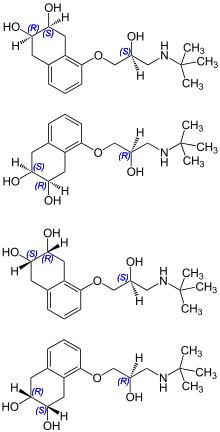
Chemistry
Nadolol is a mixture of stereoisomers. It is polar and hydrophilic, with low lipid solubility.[23]
References
- 1 2 3 4 5 6 7 "Nadolol Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 30 April 2019. Retrieved 3 March 2019.
- 1 2 3 4 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 148. ISBN 9780857113382.
- 1 2 3 Giannelli, V; Lattanzi, B; Thalheimer, U; Merli, M (2014). "Beta-blockers in liver cirrhosis". Annals of Gastroenterology. 27 (1): 20–26. PMC 3959530. PMID 24714633.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 21 October 2020. Retrieved 7 September 2020.
- ↑ "Nadolol Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 28 October 2020. Retrieved 3 March 2019.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 460. ISBN 9783527607495. Archived from the original on 2016-12-28. Retrieved 2019-03-01.
- ↑ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- ↑ "Nadolol - Drug Usage Statistics". ClinCalc. Archived from the original on 23 September 2020. Retrieved 11 April 2020.
- ↑ Nadolol entry in AccessMedicine. McGraw-Hill Global Education Holdings, LLC. Accessed 8 November 2014
- ↑ January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, et al. (2014). "2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society". Circulation. 130 (23): e199-267. doi:10.1161/CIR.0000000000000041. PMC 4676081. PMID 24682347.
- ↑ Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, et al. (2012). "Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society". Neurology. 78 (17): 1337–45. doi:10.1212/WNL.0b013e3182535d20. PMC 3335452. PMID 22529202.
- ↑ Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, et al. (2011). "Hyperthyroidism and Other Causes of Thyrotoxicosis: Management Guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists". Thyroid. 21 (6): 593–646. doi:10.1089/thy.2010.0417. PMID 21510801. Archived from the original on 2021-08-29. Retrieved 2019-12-16.
- ↑ Mazzanti A, Maragna R, Vacanti G, Monteforte N, Bloise R, et al. (15 April 2018). "Interplay Between Genetic Substrate, QTc Duration, and Arrhythmia Risk in Patients With Long QT Syndrome". Journal of the American College of Cardiology. 71 (15): 1663–1671. doi:10.1016/j.jacc.2018.01.078. PMID 29650123.
- ↑ "Corgard (nadolol) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Archived from the original on 13 May 2017. Retrieved 27 May 2017.
- ↑ "Nadolol - a beta-blocker - Corgard. High blood pressure drugs". patient.info. Archived from the original on 9 November 2017. Retrieved 27 May 2017.
- ↑ Barkley, Russell A.; Murphy, Kevin R. (27 May 2017). Attention-deficit Hyperactivity Disorder: A Clinical Workbook. Guilford Press. ISBN 9781593852276. Archived from the original on 28 August 2021. Retrieved 27 May 2017 – via Google Books.
- ↑ Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, et al. (Nov 2011). "Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology". Neurology. 77 (19): 1752–5. doi:10.1212/WNL.0b013e318236f0fd. PMC 3208950. PMID 22013182.
- 1 2 U.S. National Library of Medicine Nadolol entry in Medline Plus Archived 2016-07-05 at the Wayback Machine
- ↑ Foster NL, Newman RP, Lewitt PA, Gillespie MM, Larsen TA, et al. (October 1984). "Peripheral beta-adrenergic blockade treatment of parkinsonian tremor". Ann Neurol. 16 (4): 505–508. doi:10.1002/ana.410160412. PMID 6149724.
- ↑ "Nadolol: MedlinePlus Drug Information". www.nlm.nih.gov. Archived from the original on 5 July 2016. Retrieved 27 May 2017.
- ↑ "Nadolol Dosage Guide with Precautions - Drugs.com". drugs.com. Archived from the original on 9 November 2017. Retrieved 27 May 2017.
- ↑ "Corgard Label" (PDF). fda.gov. Archived (PDF) from the original on 17 February 2017. Retrieved 27 May 2017.
- ↑ Bragg W, Norton D, Shamsi SA (November 2008). "Optimized separation of beta-blockers with multiple chiral centers using capillary electrochromatography-mass spectrometry". J Chromatogr B. 875 (1): 304–16. doi:10.1016/j.jchromb.2008.06.028. PMC 2680439. PMID 18619928.
External links
- "Nadolol". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2019-04-10. Retrieved 2019-03-11.
| Identifiers: |
|---|