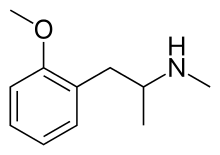
Methoxyphenamine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.035 |
| Chemical and physical data | |
| Formula | C11H17NO |
| Molar mass | 179.263 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Methoxyphenamine (trade names ASMI, Euspirol, Orthoxine, Ortodrinex, Proasma), also known as 2-methoxy-N-methylamphetamine (OMMA), is a β-adrenergic receptor agonist of the amphetamine class used as a bronchodilator.[1]
It acts as an anti-inflammatory in rats.[2]
Chemistry
Methoxyphenamine was first synthesized at the Upjohn company by Woodruff and co-workers.[3] A later synthesis by Heinzelman, from the same company, corrects the melting point given for methoxyphenamine hydrochloride in the earlier paper, and describes an improved synthetic procedure, as well as resolution of the racemic methoxyphenamine.[4]
See also
- 2-Methoxyamphetamine (OMA)
- 3-Methoxy-N-methylamphetamine (MMMA)
- 4-Methoxy-N-methylamphetamine (PMMA)
References
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ↑ Wang YH, Bai CX, Hong QY, Chen J (December 2003). "Anti-inflammatory effect of methoxyphenamine compound in rat model of chronic obstructive pulmonary disease". Acta Pharmacologica Sinica. 24 (12): 1324–7. PMID 14653967.
- ↑ Woodruff EH, Lambooy JP, Burt WE (April 1940). "Physiologically active amines. III. Secondary and tertiary β-phenylpropylamines and β-phenylisopropylamines". Journal of the American Chemical Society. 62 (4): 922–4. doi:10.1021/ja01861a060.
- ↑ Heinzelman RV (February 1953). "Physiologically active secondary amines. β-(o-Methoxyphenyl)-isopropyl-N-methylamine and related compounds". Journal of the American Chemical Society. 75 (4): 921–5. doi:10.1021/ja01100a043.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.