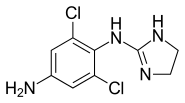
Apraclonidine
 | |
| Names | |
|---|---|
| Trade names | Iopidine |
| Other names | Apraclonidine hydrochloride |
IUPAC name
| |
| Clinical data | |
| Drug class | α2 adrenergic receptor activator[1] |
| Main uses | Glaucoma[1] |
| Side effects | Red eyes, itchiness, dry mouth, dilated pupil, runny nose, dry eyes[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Eye drop |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608005 |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 98.7% |
| Elimination half-life | 8 hours |
| Chemical and physical data | |
| Formula | C9H10Cl2N4 |
| Molar mass | 245.11 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Apraclonidine, sold under the brand name Iopidine, is a medication used to treat glaucoma.[1] Specifically it is used short term for open-angle glaucoma not controlled with other treatments.[1] It may also be used to prevent increased intraocular pressure in those undergoing certain types of laser eye surgery.[2] It is used as an eye drop.[1]
Common side effects include red eyes, itchiness, dry mouth, dilated pupil, runny nose, and dry eyes.[1] Other side effects may include allergic reactions, palpitations, depression, and dizziness.[1] Safety in pregnancy is unclear.[1] It is primarily an α2 adrenergic receptor activator and works by decreasing aqueous humour production.[1][2]
Apraclonidine was approved for medical use in the United States in 1987.[1] It is available as a generic medication.[3] In the United States 5 ml costs about 26 USD as of 2022.[3] In the United Kingdom this amount costs the NHS about £11.[2]
Medical uses
Apraclonidine is indicated for the short-term adjunctive treatment of glaucoma for patients on maximally tolerated medical therapy who require additional reduction of IOP. These patients, who are treated with apraclonidine to delay surgery, should have frequent follow-up examinations and treatment should be discontinued if the intraocular pressure rises significantly.
Apraclonidine may be useful in the diagnosis of Horner's syndrome. In Horner's syndrome, the sympathetic innervation to the pupillary dilator muscle is lost. The affected pupil is thus miotic and the pupillary dilator responds to denervation by increasing α1 receptors. Apraclonidine is useful in this case due to its weak α1-adrenergic properties. When applied to the denervated (and thus hyper-sensitive) pupillary dilator muscle, a super-normal dilatory response is generated in which the pupil dilates to a degree greater than that which would be seen in a non-denervated muscle. This causes the reversal of anisocoria that is characteristic of Horner's.
Topical apraclonidine can also decrease IOP in glaucoma patients by increasing trabecular outflow, in a similar way to clonidine,[4] but without the cardiovascular side effects. Apraclonidine has been compared with other treatments such as brimonidine and pilocarpine in preventing IOP spikes after laser trabeculoplasty.[5] The results did not show significant differences in the reduction of IOP for apraclonidine, when compared to brimonidine or pilocarpine.[5]
Dosage
Topical apraclonidine is administered at a concentration of 1% for the prevention and treatment of post-surgical intraocular pressure (IOP) elevation and 0.5% for short-term adjunctive therapy in people on maximally tolerated medical therapy who require additional reduction of IOP. One drop is usually added one hour prior to laser eye surgery and another drop is given after the procedure is complete.
References
- 1 2 3 4 5 6 7 8 9 10 11 "Apraclonidine Monograph for Professionals". Drugs.com. Archived from the original on 25 January 2021. Retrieved 15 January 2022.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1232. ISBN 978-0857114105.
- 1 2 "Apraclonidine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 10 July 2016. Retrieved 15 January 2022.
- ↑ Toris, C. B.; Tafoya, M. E.; Camras, C. B.; Yablonski, M. E. (1995). "Effects of Apraclonidine on Aqueous Humor Dynamics in Human Eyes". Ophthalmology. 102 (3): 456–61. doi:10.1016/S0161-6420(95)31000-7. PMID 7891985.
- 1 2 Zhang L, Weizer JS, Musch DC (2017). "Perioperative medications for preventing temporarily increased intraocular pressure after laser trabeculoplasty". Cochrane Database Syst Rev. 2: CD010746. doi:10.1002/14651858.CD010746.pub2. PMC 5477062. PMID 28231380.
External links
| Identifiers: |
|---|
- Iopidine prescribing information Archived 2021-06-30 at the Wayback Machine (from the FDA website)