Unoprostone
 | |
| Clinical data | |
|---|---|
| Trade names | Rescula |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 14 min |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.227.145 |
| Chemical and physical data | |
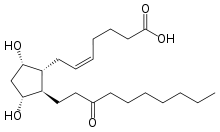
| Formula | C22H38O5 |
| Molar mass | 382.541 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Unoprostone (INN) is a prostaglandin analogue. Its isopropyl ester, unoprostone isopropyl, was marketed under the trade name Rescula for the management of open-angle glaucoma and ocular hypertension.[1][2]
It was approved by the Food and Drug Administration in 2000.[3]
In 2009, Sucampo Pharmaceuticals acquired the rights to the drug in the U.S. and Canada.[4]
In 2015, the drug was discontinued in the U.S.
References
- ↑ Micromedex Detailed Consumer Information
- ↑ Fung DS, Whitson JT (2014). "An evidence-based review of unoprostone isopropyl ophthalmic solution 0.15% for glaucoma: place in therapy". Clinical Ophthalmology. Auckland, N.Z. 8: 543–54. doi:10.2147/OPTH.S41562. PMC 3958522. PMID 24648719.
- ↑ "Drug Approval Package". Food and Drug Administration.
- ↑ "Sucampo Pharmaceuticals, Inc. Acquires Rights to Rescula for U.S. and Canada" (Press release). Business Wire. April 24, 2009.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.