Apricoxib
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
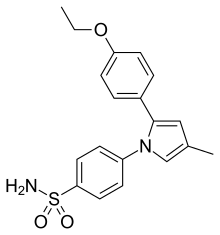
| Formula | C19H20N2O3S |
| Molar mass | 356.44 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Apricoxib is an experimental anticancer drug and nonsteroidal anti-inflammatory drug (NSAID).[1] It is a COX-2 inhibitor which is intended to improve standard therapy response in molecularly-defined models of pancreatic cancer.[2] It was also studied in clinical trials for non-small-cell lung cancer.[3] Development was abandoned in 2015 due to poor clinical trial results.[4]
See also
- Tilmacoxib
- Cimicoxib
- NS-398
- Celecoxib
References
- ↑ "Apricoxib (Code C74021)". NCI Thesaurus. National Cancer Institute.
- ↑ Kirane A, Toombs JE, Ostapoff K, Carbon JG, Zaknoen S, Braunfeld J, et al. (September 2012). "Apricoxib, a novel inhibitor of COX-2, markedly improves standard therapy response in molecularly defined models of pancreatic cancer". Clinical Cancer Research. 18 (18): 5031–42. doi:10.1158/1078-0432.CCR-12-0453. PMC 3777527. PMID 22829202.
- ↑ Edelman MJ, Tan MT, Fidler MJ, Sanborn RE, Otterson G, Sequist LV, et al. (January 2015). "Randomized, double-blind, placebo-controlled, multicenter phase II study of the efficacy and safety of apricoxib in combination with either docetaxel or pemetrexed in patients with biomarker-selected non-small-cell lung cancer". Journal of Clinical Oncology. 33 (2): 189–94. doi:10.1200/JCO.2014.55.5789. PMC 4890680. PMID 25452446.
- ↑ "Apricoxib". Adis Insight. Springer Nature Switzerland AG.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.