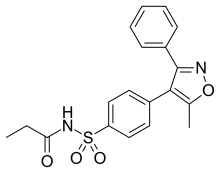
Parecoxib
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category |
|
| Routes of administration | Intravenous and intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 98% |
| Metabolism | Hepatic to valdecoxib and propionic acid CYP extensively involved (mainly CYP3A4 and 2C9) |
| Elimination half-life | 22 minutes (parecoxib) 8 hours (valdecoxib) |
| Excretion | Renal (70%, metabolites) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.078 |
| Chemical and physical data | |
| Formula | C19H18N2O4S |
| Molar mass | 370.42 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Parecoxib, sold under the brand name Dynastat among others, is a water-soluble and injectable prodrug of valdecoxib. Parecoxib is a COX2 selective inhibitor. It is injectable. It is approved through much of Europe for short term perioperative pain control.
It was patented in 1996 and approved for medical use in 2002.[1]
Approval
In 2005, the U.S. Food and Drug Administration (FDA) issued a letter of non-approval for parecoxib in the United States. No reasons were ever documented publicly for the non-approval, although one study noted increased occurrences of heart attacks following cardiac bypass surgery compared to placebo when high doses of parecoxib were used to control pain after surgery. Importantly, rare but severe allergic reactions (Stevens–Johnson syndrome, Lyell syndrome) have been described with valdecoxib, the molecule to which parecoxib is converted.[2] The drug is not approved for use after cardiac surgery in Europe.
All anti-inflammatory medications in the U.S. carry the same warning regarding skin reactions, and none are approved for use during CABG surgery, so the reason for the FDA denying the approval of parecoxib remains unknown, but was likely related to political pressure from the US Congress to not approve another COX-2 selective inhibitor in the wake of the Vioxx affair. No COX-2 selective inhibitor has been approved in the US since that time, regardless of the safety profile of parecoxib in Europe. Efforts to find out the scientific rationale, or more likely the lack thereof, that the FDA used to justify the non-approval of parecoxib in the USA have proven futile due to secrecy issues.[3][4]
The political motivation to not approve parecoxib was further supported by a 2017 pooled analysis of safety data in 28 published studies, which showed after 69,567,300 units of parecoxib, skin rash and cardiac complications were minimal, if at all, different from placebo.[5]
Parecoxib, along with other COX-2 selective inhibitors, celecoxib, valdecoxib, and mavacoxib, were discovered by a team at the Searle division of Monsanto led by John Talley.[6][7]
Parecoxib is the first parenteral COX-2 selective inhibitor available for clinical use in pain management. It is well known from previous clinical trials that its peak serum concentrations occur about thirty minutes after intravenous (IV) administration and one hour after intramuscular (IM) injection. Its first perceptible analgesic effect occurs within seven to thirteen minutes, with clinically meaningful analgesia demonstrated within twenty-three to thirty-nine minutes and a peak effect within two hours following administration of single doses of 40 mg by IV or IM injection.[8]
See also
References
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 522. ISBN 9783527607495.
- ↑ Health "Association of Bextra (Valdecoxib) with Serious Adverse Drug Reactions". Health Canada. April 21, 2005.
- ↑ Gajraj NM (2007). "COX-2 inhibitors celecoxib and parecoxib: valuable options for postoperative pain management". Current Topics in Medicinal Chemistry. 7 (3): 235–49. doi:10.2174/156802607779941323. PMID 17305567.
- ↑ Kiehl S (March 13, 2005). "Secrecy on the Rise". The Baltimore Sun.
- ↑ Schug SA, Parsons B, Li C, Xia F (2017). "The safety profile of parecoxib for the treatment of postoperative pain: a pooled analysis of 28 randomized, double-blind, placebo-controlled clinical trials and a review of over 10 years of postauthorization data". Journal of Pain Research. 10: 2451–2459. doi:10.2147/jpr.s136052. PMC 5644539. PMID 29066931.
- ↑ Langreth R (June 23, 2003). "The Chemical Cobbler". Forbes.
- ↑ "Dr. John Talley: 2001 St. Louis Awardee" (PDF). Chemical Bond. St. Louis Section, American Chemical Society. 52 (5): 2. May 2001. Archived from the original (PDF) on 15 April 2018.
- ↑ Mulita, Francesk; Karpetas, Georgios; Liolis, Elias; Vailas, Michail; Tchabashvili, Levan; Maroulis, Ioannis (2021). "Comparison of analgesic efficacy of acetaminophen monotherapy versus acetaminophen combinations with either pethidine or parecoxib in patients undergoing laparoscopic cholecystectomy: a randomized prospective study". Medicinski Glasnik Ljekarske komore Zenicko-dobojskog kantona. 18 (1). doi:10.17392/1245-21. ISSN 1840-0132. PMID 33155461.
Further reading
- Villasís-Keever MA, Rendón-Macías ME, Escamilla-Núñez A (2009). "[Systematic review to assess the effectiveness and safety of parecoxib]". Acta Ortopedica Mexicana (in European Spanish). 23 (6): 342–50. PMID 20377000.
- Lloyd R, Derry S, Moore RA, McQuay HJ (April 2009). "Intravenous or intramuscular parecoxib for acute postoperative pain in adults". The Cochrane Database of Systematic Reviews (2): CD004771. doi:10.1002/14651858.CD004771.pub4. PMC 6540719. PMID 19370610.