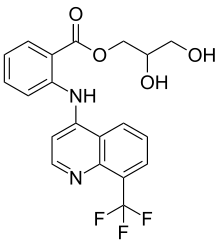
Floctafenine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.696 |
| Chemical and physical data | |
| Formula | C20H17F3N2O4 |
| Molar mass | 406.361 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Floctafenine is a nonsteroidal anti-inflammatory drug (NSAID).
Synthesis
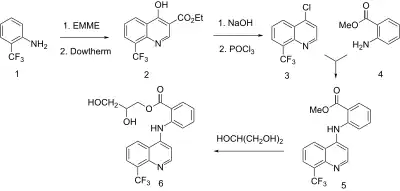
The scheme involves first the conversion of ortho-trifluoromethyl aniline (1) to a quinolol. The compound is thus condensed with EMME (Ethoxy Methylene Malonic Diethyl Ester) and cyclized thermally (2). That intermediate is then saponified; the resulting acid is decarboxylated and finally converted to the 4-chloroquinoline (3) by reaction with phosphorus oxychloride. The displacement of chlorine with methyl anthranilate (4) then affords the coupled intermediate (5). An ester interchange of that product with glycerol leads to the glyceryl ester. There is thus obtained the NSAID floctafenine.
See also
- Glafenine, a chemically related NSAID
References
- ↑ DE 1815467A1, Allais, André & Meier, Jean, "Neue Chinolidinderivate und Verfahren zu ihrer Herstellung [Novel quinolidine derivatives and processes for their preparation]", published 1969-07-24, assigned to Roussel-Uclaf
- ↑ Mouzin G, Cousse H, Autin JM (1980). "A New, Convenient Synthesis of Glafenine and Floctafenine". Synthesis. 1980: 54–55. doi:10.1055/s-1980-28923.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.