Argatroban
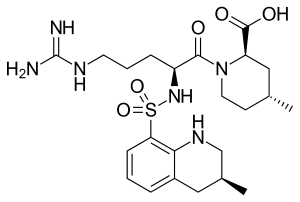 | |
| Names | |
|---|---|
IUPAC name
| |
| Clinical data | |
| Drug class | Direct thrombin inhibitor[1] |
| Main uses | Prevent and treat blood clots in people with heparin-induced thrombocytopenia (HIT)[1][2] |
| Side effects | Low blood pressure, fever, diarrhea, shortness of breath, headache[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 100% (intravenous) |
| Protein binding | 54% |
| Metabolism | hepatic |
| Elimination half-life | 39 and 51 minutes |
| Chemical and physical data | |
| Formula | C23H36N6O5S |
| Molar mass | 508.64 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Argatroban is a medication used to prevent and treat blood clots in people who have heparin-induced thrombocytopenia (HIT).[1][2] It may also be used in people undergoing percutaneous coronary intervention (PCI) with HIT.[2] It is given by gradual injection into a vein.[1]
Common side effects include low blood pressure, fever, diarrhea, shortness of breath, and headache.[2] Other side effects may include bleeding.[2] It should not be used in people with severe liver problems.[2] Safety in pregnancy is unclear.[2] It is an anticoagulant, specifically a direct thrombin inhibitor.[1][4]
Argatroban was approved for medical use in the United States in 2000.[2] It is available as a generic medication.[1] In the United Kingdom 50 mg costs about £50 as of 2021.[1] This amount in the United States is about 110 USD.[5]
Medical uses
In 2002, it was approved for use during percutaneous coronary interventions in patients who have HIT or are at risk for developing it. In 2012, it was approved by the MHRA in the UK for anticoagulation in patients with heparin-induced thrombocytopenia Type II (HIT) who require parenteral antithrombotic therapy.[6]
Dosage
It is generally started at 2 micrograms/kg per minute.[1] This is than adjusted up to 10 micrograms/kg per minute based on the activated partial thromboplastin.[1]
Transition to warfarin
Argatroban is used as an anticoagulant in individuals with thrombosis and heparin-induced thrombocytopenia. Often these individuals require long-term anticoagulation. If warfarin is chosen as the long-term anticoagulant, this poses particular challenges due to the falsely elevated prothrombin time and INR caused by argatroban. The combination of argatroban and warfarin may raise the INR to greater than 5.0 without a significant increased risk of bleeding complications.[7] One solution to this problem is to measure the chromogenic factor X level. A level < 40-45% typically indicates that the INR will be therapeutic (2-3) when the argatroban is discontinued.
Pharmacology
Argatroban is metabolized in the liver and has a half-life of about 50 minutes. It is monitored by PTT. Because of its hepatic metabolism, it may be used in people with renal dysfunction. (This is in contrast to lepirudin, a direct thrombin inhibitor that is primarily renally cleared).
References
- 1 2 3 4 5 6 7 8 9 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 147. ISBN 978-0857114105.
- 1 2 3 4 5 6 7 8 9 "DailyMed - ARGATROBAN injection". dailymed.nlm.nih.gov. Archived from the original on 16 January 2022. Retrieved 16 January 2022.
- ↑ "Argatroban injection, solution". DailyMed. Archived from the original on 10 June 2021. Retrieved 10 June 2021.
- ↑ Di Nisio M, Middeldorp S, Buller HR (2005). "Direct thrombin inhibitors". N Engl J Med. 353 (10): 1028–40. doi:10.1056/NEJMra044440. PMID 16148288. Archived from the original on 2021-11-05. Retrieved 2021-06-13.
- ↑ "Argatroban Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 22 January 2021. Retrieved 16 January 2022.
- ↑ "UK launch for Mitsubishi's blood thinner Exembol". 3 July 2012. Archived from the original on 4 March 2016. Retrieved 13 June 2021.
- ↑ Hursting MJ, Lewis BE, Macfarlane DE (2005). "Transitioning from argatroban to warfarin therapy in patients with heparin-induced thrombocytopenia". Clin Appl Thromb Hemost. 11 (3): 279–87. doi:10.1177/107602960501100306. PMID 16015413.
External links
| External sites: |
|
|---|---|
| Identifiers: |