Nadroparin
 | |
| Names | |
|---|---|
| Trade names | Fraxiparin, Fraxiparine, Fraxodi, others |
| Other names | Nadroparin calcium[1] |
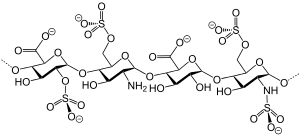
IUPAC name
| |
| Clinical data | |
| Drug class | Low molecular weight heparin (LMWH)[1] |
| Main uses | Prevent and treat blood clots[1] |
| Side effects | Bruising at the site of injection, bleeding, heparin induced thrombocytopenia, high potassium[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Subcutaneous injection (except for haemodialysis) |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 89% (SC dose) |
| Elimination half-life | 3.7 hours (SC dose) |
| Excretion | clearance 21.4mL/min (+/- 7) |
| Chemical and physical data | |
| Molar mass | 4300 g/mol |
Nadroparin, sold under the brand name Fraxiparin among others, is a medication used to prevent and treat blood clots, including deep vein thrombosis (DVT) and pulmonary embolism.[1] It may be used following surgery, in people in hospital who are not moving, and as with hemodialysis.[1][2] It is given by injection under the skin.[1]
Common side effects include bruising at the site of injection and bleeding.[1][2] Other side effects may include heparin induced thrombocytopenia and high potassium.[2] In those with kidney problems, lower doses may be required.[2] It is a low molecular weight heparin (LMWH) which works by attaching to plasma protein anti-thrombin III.[1][2] It is made from unfractionated heparin.[2]
Nadroparin was approved for medical use in Australia in 1995.[2] It is on the World Health Organization's List of Essential Medicines as an alternative to enoxaparin.[3] It is available in a number of European countries and Canada but not the United States.[4][5]
History
Nadroparin was developed by Sanofi-Synthélabo.
References
- 1 2 3 4 5 6 7 8 Davis, R; Faulds, D (April 1997). "Nadroparin calcium. A review of its pharmacology and clinical use in the prevention and treatment of thromboembolic disorders". Drugs & aging. 10 (4): 299–322. doi:10.2165/00002512-199710040-00006. PMID 9108990.
- 1 2 3 4 5 6 7 8 "Fraxiparine" (PDF). TGA. Archived (PDF) from the original on 16 September 2023. Retrieved 13 September 2023.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ "List of nationally authorised medicinal products" (PDF). EMA. Archived (PDF) from the original on 30 March 2023. Retrieved 9 September 2023.
- ↑ Frontera, Walter R.; Silver, Julie K. (26 September 2018). Essentials of Physical Medicine and Rehabilitation E-Book: Musculoskeletal Disorders, Pain, and Rehabilitation. Elsevier Health Sciences. p. 715. ISBN 978-0-323-54966-0. Archived from the original on 16 September 2023. Retrieved 13 September 2023.
External links
| Identifiers: |
|---|
- "NCI Drug Dictionary". National Cancer Institute. 2 February 2011. Archived from the original on 10 August 2019. Retrieved 2 April 2019.