Tenecteplase
| Names | |
|---|---|
| Trade names | TNKase, Metalyse, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Tissue plasminogen activator (tPA)[1] |
| Main uses | ST elevation myocardial infarction (STEMI), pulmonary embolism[1] |
| Side effects | Bleeding[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Typical dose | 30 to 50 mg[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Excretion | Liver |
| Chemical and physical data | |
| Formula | C2561H3919N747O781S40 |
| Molar mass | 58951.37 g·mol−1 |
Tenecteplase (TNK), sold under the trade names TNKase among others, is a medication used to treat ST elevation myocardial infarction or pulmonary embolism with low blood pressure.[1][3] It may also be used for stroke.[3] It is given by injection into a vein.[1]
Common side effects include bleeding such as nosebleeds, gastrointestinal bleeding, bruising, or blood in the urine.[1][2] It should not be used by those with anaphylaxis to gentamicin.[2] It is a tissue plasminogen activator (tPA) produced by recombinant DNA technology.[1] It works by dissolving blood clots.[2]
Tenecteplase was approved for medical use in the United States in 2000 and Europe in 2001.[1][2] In the United Kingdom it costs the NHS about £600 per dose as of 2021.[4] This amount in the United States costs about 6,500 USD.[5]
Medical uses
It is most commonly used for ST elevation myocardial infarction.[1] It may also be used for pulmonary embolism with low blood pressure and to treat stroke.[3][1]
The American Heart Association/American Stroke Association 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke supports considering tenecteplase over alteplase in people without contraindication to intravenous thrombolytics.[6]
Dosage
For STEMI the typical dose is 30 to 50 mg.[1] It is given over 5 to 10 seconds.[1][2] A similar dose may be used is massive pulmonary embolism.[7]
The amount of 10,000 units is equal to 50 mg.[8]
Mechanism of action
It binds to the fibrin component of the thrombus (blood clot) and selectively converts thrombus-bound plasminogen to plasmin, which degrades the fibrin matrix of the thrombus. Tenecteplase has a higher fibrin specificity and greater resistance to inactivation by its endogenous inhibitor (PAI-1) compared to native t-PA.
Pharmacokinetics
Distribution: approximates plasma volume
Metabolism: Primarily hepatic
Half-life elimination: Biphasic: Initial: 20–24 minutes; Terminal: 90–130 minutes
Excretion: Clearance: Plasma: 99-119 mL/minute
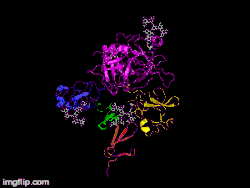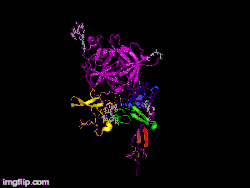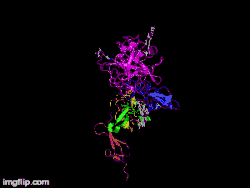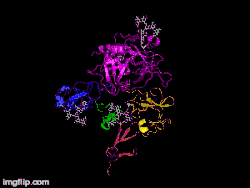 Here is TNK-tPA. It is very similar to t-PA, but the glycosylation occurring in Kringle 1 is manipulated. The mutation T103N means that glycosylation occurs at that point. The mutation N117E means that the high mannose sugar residue is absent at that point
Here is TNK-tPA. It is very similar to t-PA, but the glycosylation occurring in Kringle 1 is manipulated. The mutation T103N means that glycosylation occurs at that point. The mutation N117E means that the high mannose sugar residue is absent at that point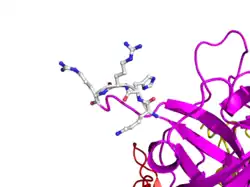 In human t-PA, the amino acids at position 296-299 are Lysine, Histidine, and two Arginines.
In human t-PA, the amino acids at position 296-299 are Lysine, Histidine, and two Arginines.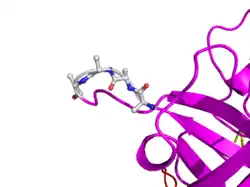 In TNK-tPA, these amino acids have been replaced by four Alanines. This mutation is responsible for increased resistance to PAI-1.
In TNK-tPA, these amino acids have been replaced by four Alanines. This mutation is responsible for increased resistance to PAI-1.
Manufacture
It is a tissue plasminogen activator (tPA) produced by recombinant DNA technology using an established mammalian cell line (Chinese hamster ovary cells). Tenecteplase is a 527 amino acid glycoprotein developed by introducing the following modifications to the complementary DNA (cDNA) for natural human tPA: a substitution of threonine 103 with asparagine, and a substitution of asparagine 117 with glutamine, both within the kringle 1 domain, and a tetra-alanine substitution at amino acids 296–299 in the protease domain.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 "Tenecteplase Monograph for Professionals". Drugs.com. Archived from the original on 15 August 2019. Retrieved 19 September 2021.
- 1 2 3 4 5 6 "Metalyse". Archived from the original on 11 November 2020. Retrieved 19 September 2021.
- 1 2 3 Burgos, Adrian M.; Saver, Jeffrey L. (August 2019). "Evidence that Tenecteplase Is Noninferior to Alteplase for Acute Ischemic Stroke: Meta-Analysis of 5 Randomized Trials". Stroke. 50 (8): 2156–2162. doi:10.1161/STROKEAHA.119.025080.
- ↑ BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 231. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "TNKase Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 January 2021. Retrieved 19 September 2021.
- ↑ "Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association". www.ahajournals.org. doi:10.1161/str.0000000000000211. Archived from the original on 2020-11-22. Retrieved 2021-05-13.
- ↑ Martin, C; Sobolewski, K; Bridgeman, P; Boutsikaris, D (December 2016). "Systemic Thrombolysis for Pulmonary Embolism: A Review". P & T : a peer-reviewed journal for formulary management. 41 (12): 770–775. PMID 27990080.
- ↑ "Metalyse 10,000 units - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Archived from the original on 2 March 2021. Retrieved 19 September 2021.
External links
| Identifiers: |
|---|