Acetazolamide
 | |
 | |
| Names | |
|---|---|
| Trade names | Diamox, Diacarb, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Diuretic (carbonic anhydrase inhibitor)[1] |
| Main uses | Glaucoma, epilepsy, altitude sickness, periodic paralysis, idiopathic intracranial hypertension, heart failure[1][2] |
| Side effects | Numbness, ringing in the ears, loss of appetite, vomiting, sleepiness[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth or intravenous |
| Defined daily dose | 0.75 g[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 70–90%[4] |
| Metabolism | None[4] |
| Elimination half-life | 2–4 hours[4] |
| Excretion | Urine (90%)[4] |
| Chemical and physical data | |
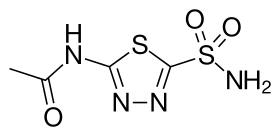
| Formula | C4H6N4O3S2 |
| Molar mass | 222.24 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 258 to 259 °C (496 to 498 °F) |
SMILES
| |
InChI
| |
Acetazolamide, sold under the trade name Diamox among others, is a medication used to treat glaucoma, epilepsy, altitude sickness, periodic paralysis, idiopathic intracranial hypertension (raised brain pressure of unclear cause), and heart failure.[1][2] It may be used long term for the treatment of open angle glaucoma and short term for acute angle closure glaucoma until surgery can be carried out.[5] It is taken by mouth or injection into a vein.[1]
Common side effects include numbness, ringing in the ears, loss of appetite, vomiting, and sleepiness.[1] It is not recommended in those with significant kidney problems, liver problems, or who are allergic to sulfonamides.[1][5] Acetazolamide is in the diuretic and carbonic anhydrase inhibitor families of medication.[1] It works by decreasing the amount of hydrogen ions and bicarbonate in the body.[1]
Acetazolamide came into medical use in 1952.[6] It is on the World Health Organization's List of Essential Medicines.[7] Acetazolamide is available as a generic medication.[1] The wholesale cost in the developing world is about US$1.40–16.93 per month.[8] In the United States the wholesale cost is about US$125.34 per month.[9]
Medical uses
It is used in the treatment of glaucoma, drug-induced edema, heart failure-induced edema, epilepsy and in reducing intraocular pressure after surgery.[10][11] It has also been used in the treatment of altitude sickness,[12] Ménière's disease, increased intracranial pressure and neuromuscular disorders.[13]
In epilepsy, the main use of acetazolamide is in menstrual-related epilepsy and as an add on to other treatments in refractory epilepsy.[10][14] It has been demonstrated in drug trials to relieve symptoms associated with dural ectasia in individuals with Marfan's Syndrome.[15] A 2012 review and meta-analysis found that there was "limited supporting evidence" but that acetazolamide "may be considered" for the treatment of central (as opposed to obstructive) sleep apnea.[16]
It has also been used to prevent methotrexate-induced kidney damage by alkalinalizing the urine, hence speeding up methotrexate excretion by increasing its solubility in urine.[13][17] There is some evidence to support its use to prevent hemiplegic migraine.[18]
High altitude sickness
In the prevention or treatment of mountain sickness, acetazolamide forces the kidneys to excrete bicarbonate, the conjugate base of carbonic acid. By increasing the amount of bicarbonate excreted in the urine, the blood becomes more acidic.[13] As the body equates acidity of the blood to its CO2 concentration, artificially acidifying the blood fools the body into thinking it has an excess of CO2, and it excretes this perceived excess CO2 by deeper and faster breathing, which in turn increases the amount of oxygen in the blood.[19][20] Acetazolamide is not an immediate cure for acute mountain sickness; rather, speeds up (or, when taking before traveling, forces the body to early start) part of the acclimatization process which in turn helps to relieve symptoms.[21] Acetazolamide is still effective if started early in the course of mountain sickness. As prevention, it is started one day before travel to altitude and continued for the first 2 days at altitude.[22]
Pregnancy and breastfeeding
Acetazolamide is pregnancy category B3 in Australia, which means that studies in rats, mice and rabbits in which acetazolamide was given intravenously or orally caused an increased risk of fetal malformations, including defects of the limbs.[11] Despite this, there is insufficient evidence from studies in humans to either support or discount this evidence.[11]
Limited data are available on the effects of nursing mothers taking acetazolamide. Therapeutic doses create low levels in breast milk and are not expected to cause problems in infants.[23]
Dosage
The defined daily dose is 0.75 gram by mouth or by injection.[3]
Side effects
Common adverse effects of acetazolamide include the following: paraesthesia, fatigue, drowsiness, depression, decreased libido, bitter or metallic taste, nausea, vomiting, abdominal cramps, diarrhea, black feces, polyuria, kidney stones, metabolic acidosis and electrolyte changes (hypokalemia, hyponatremia).[10] Whereas less common adverse effects include Stevens–Johnson syndrome, anaphylaxis and blood dyscrasias.[10]
Contraindications
Contraindications include:[11]
- Hyperchloremic acidosis
- Hypokalemia (low blood potassium)
- Hyponatremia (low blood sodium)
- Adrenal insufficiency
- Impaired kidney function
- Hypersensitivity to acetazolamide or other sulfonamides.
- Marked liver disease or impairment of liver function, including cirrhosis because of the risk of development of hepatic encephalopathy. Acetazolamide decreases ammonia clearance.
Interactions
It is possible that it might interact with:[11]
- Amphetamines, because it increases the pH of the renal tubular urine, hence reducing the clearance of amphetamines.
- Other carbonic anhydrase inhibitors — potential for additive inhibitory effects on carbonic anhydrase and hence potential for toxicity.
- Ciclosporin, may increase plasma levels of ciclosporin.
- Antifolates such as trimethoprim, methotrexate, pemetrexed and raltitrexed.
- Hypoglycemics, acetazolamide can both increase or decrease blood glucose levels.
- Lithium, increases excretion, hence reducing therapeutic effect.
- Methenamine compounds, reduces the urinary excretion of methenamines.
- Phenytoin, reduces phenytoin excretion, hence increasing the potential for toxicity.
- Primidone, reduces plasma levels of primidone. Hence reducing anticonvulsant effect.
- Quinidine, reduces urinary excretion of quinidine, hence increasing the potential for toxicity.
- Salicylates, potential for severe toxicity.
- Sodium bicarbonate, potential for kidney stone formation.
- Anticoagulants, cardiac glycosides, may have their effects potentiated by acetazolamide.
Mechanism of action


Acetazolamide is a carbonic anhydrase inhibitor, hence causing the accumulation of carbonic acid.[13] Carbonic anhydrase is an enzyme found in red blood cells and many other tissues that catalyses the following reaction:[24]
- H2CO3 ⇌ H2O + CO2
hence lowering blood pH, by means of the following reaction that carbonic acid undergoes:[25]
- H2CO3 ⇌ HCO3− + H+
which has a pKa of 6.3.[25]
The mechanism of diuresis involves the proximal tubule of the kidney. The enzyme carbonic anhydrase is found here, allowing the reabsorption of bicarbonate, sodium, and chloride. By inhibiting this enzyme, these ions are excreted, along with excess water, lowering blood pressure, intracranial pressure, and intraocular pressure. By excreting bicarbonate, the blood becomes acidic, causing compensatory hyperventilation with deep respiration (Kussmaul respiration), increasing levels of oxygen and decreasing levels of carbon dioxide in the blood.[26]
In the eye this results in a reduction in aqueous humour.[11]
Bicarbonate (HCO3−) has a pKa of 10.3 with carbonate (CO32−), far further from physiologic pH (7.35–7.45), and so it is more likely to accept a proton than to donate one, but it is also far less likely for it to do either, thus bicarbonate will be the major species at physiological pH.
Under normal conditions in the proximal convoluted tubule of the kidney, most of the carbonic acid (H2CO3) produced intracellularly by the action of carbonic anhydrase quickly dissociates in the cell to bicarbonate (HCO3−) and an H+ ion (a proton), as previously mentioned. The bicarbonate (HCO3−) exits at the basal portion of the cell via sodium (Na+) symport and chloride (Cl−) antiport and re-enters circulation, where it may accept a proton if blood pH decreases, thus acting as a weak, basic buffer. The remaining H+ left over from the intracellular production of carbonic acid (H2CO3) exits the apical (urinary lumen) portion of the cell by Na+ antiport, acidifying the urine. There, it may join with another bicarbonate (HCO3−) that dissociated from its H+ in the lumen of the urinary space only after exiting the proximal convoluted kidney cells/glomerulus as carbonic acid (H2CO3) because bicarbonate (HCO3−) itself can not diffuse across the cell membrane in its polar state. This will replenish carbonic acid (H2CO3) so that it then may be reabsorbed into the cell as itself or CO2 and H2O (produced via a luminal carbonic anhydrase). As a result of this whole process, there is a greater net balance of H+ in the urinary lumen than bicarbonate (HCO3−), and so this space is more acidic than physiologic pH. Thus, there is an increased likelihood that any bicarbonate (HCO3−) that was left over in the lumen diffuses back into the cell as carbonic acid, CO2, or H2O.
In short, under normal conditions, the net effect of carbonic anhydrase in the urinary lumen and cells of the proximal convoluted tubule is to acidify the urine and transport bicarbonate (HCO3−) into the body. Another effect is excretion of Cl− as it is needed to maintain electroneutrality in the lumen, as well as the reabsorption of Na+ into the body.
Thus, by disrupting this process with acetazolamide, urinary Na+ and bicarbonate (HCO3−) are increased, and urinary H+ and Cl− are decreased. Inversely, serum Na+ and bicarbonate (HCO3−) are decreased, and serum H+ and Cl− are increased. H2O generally follows sodium, and so this is how the clinical diuretic effect is achieved, which reduces blood volume and thus preload on the heart to improve contractility and reduce blood pressure, or achieve other desired clinical effects of reduced blood volume such as reducing edema or intracranial pressure.[27]
History
Acetazolamide was first produced in 1950 and then used for epilepsy in 1952.[28] It was marketed as diamox in 1954 and found to be useful in wide angle glaucoma.[29] As a diuretic, it was replaced by the introduction of chlorothiazide in 1957.[29]
References
- 1 2 3 4 5 6 7 8 9 10 "Acetazolamide". The American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- 1 2 Smith, SV; Friedman, DI (30 July 2017). "The Idiopathic Intracranial Hypertension Treatment Trial: A Review of the Outcomes". Headache. 57 (8): 1303–1310. doi:10.1111/head.13144. PMID 28758206.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 24 November 2020. Retrieved 16 September 2020.
- 1 2 3 4 "Diamox Sequels (acetazolamide) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 13 April 2014. Retrieved 10 April 2014.
- 1 2 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 439. hdl:10665/44053. ISBN 9789241547659.
- ↑ Sneader, Walter (2005). Drug Discovery: A History. John Wiley & Sons. p. 390. ISBN 9780471899792. Archived from the original on 28 December 2016.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Acetazolamide". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 8 December 2016.
- ↑ "NADAC as of 2016-12-07 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 21 December 2016. Retrieved 28 December 2016.
- 1 2 3 4 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- 1 2 3 4 5 6 "Product Information Diamox Acetazolamide Tablets" (PDF). TGA eBusiness Services. Aspen Pharma Pty Ltd. 25 February 2005. Archived from the original on 4 November 2016. Retrieved 10 April 2014.
- ↑ Low, EV; Avery, AJ; Gupta, V; Schedlbauer, A; Grocott, MP (October 2012). "Identifying the lowest effective dose of acetazolamide for the prophylaxis of acute mountain sickness: systematic review and meta-analysis" (PDF). BMJ. 345: e6779. doi:10.1136/bmj.e6779. PMC 3475644. PMID 23081689. Archived from the original on 13 April 2014. Retrieved 10 April 2014.
- 1 2 3 4 Brayfield, A, ed. (7 January 2014). "Acetazolamide". Martindale: The Complete Drug Reference. Pharmaceutical Press. Archived from the original on 27 August 2021. Retrieved 10 April 2014.
- ↑ Reiss, WG; Oles, KS (May 1996). "Acetazolamide in the treatment of seizures". The Annals of Pharmacotherapy. 30 (5): 514–9. doi:10.1177/106002809603000515. PMID 8740334.
- ↑ Scoliosis Research Society (27 November 2006). "Dural Ectasia in the Marfan Spine: Symptoms and Treatment.also it's been used in high-altitude mountain sickness". SpineUniverse. Archived from the original on 26 September 2007. Retrieved 15 November 2007.
- ↑ Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, Kristo DA, Mallea JM, Rowley JA, Zak RS, Tracy SL (January 2012). "The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses". Sleep. 35 (1): 17–40. doi:10.5665/sleep.1580. PMC 3242685. PMID 22215916.
- ↑ Shamash, J; Earl, H; Souhami, R (1991). "Acetazolamide for alkalinisation of urine in patients receiving high-dose methotrexate". Cancer Chemotherapy and Pharmacology. 28 (2): 150–1. doi:10.1007/BF00689708. PMID 2060085.
- ↑ Russell, Michael Bjørn; Ducros, Anne (May 2011). "Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management". The Lancet Neurology. 10 (5): 457–470. doi:10.1016/S1474-4422(11)70048-5. PMID 21458376.
- ↑ "Altitude.org". 2004. Archived from the original on 8 February 2009. Retrieved 5 June 2009.
- ↑ Leaf DE, Goldfarb DS (April 2007). "Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness". J. Appl. Physiol. 102 (4): 1313–22. doi:10.1152/japplphysiol.01572.2005. PMID 17023566.
- ↑ Muza, SR; Fulco, CS; Cymerman, A (2004). "Altitude Acclimatization Guide". US Army Research Inst. Of Environmental Medicine Thermal and Mountain Medicine Division Technical Report (USARIEM–TN–04–05). Archived from the original on 23 April 2009. Retrieved 5 March 2009.
- ↑ World Health Organization. "International Travel and Health 2012" (PDF). Archived (PDF) from the original on 19 March 2017. Retrieved 27 January 2017.
- ↑ "LactMed: Acetazolamide". National Institutes of Health. Archived from the original on 11 October 2017. Retrieved 10 October 2017.
- ↑ Dutta, S; Goodsell, D (January 2004). "January 2004: Carbonic Anhydrase" (PDF). RCSB PDB Protein Data Bank. Archived (PDF) from the original on 14 May 2013. Retrieved 10 April 2014.
- 1 2 Larsen, D. "Carbonic Anhydrase 2". UC Davis Chemwiki. University of California. Retrieved 10 April 2014.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 13 December 2014. Retrieved 8 December 2014.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Koeppen BM. The kidney and acid-base regulation. Adv Physiol Educ. 2009;33(4):275-81. Retrieved from "Archived copy". Archived from the original on 20 April 2016. Retrieved 31 March 2016.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Chapman, Kevin; Wheless, James W.; Stafstrom, Carl E. (2010). "22. Special treatments in epilepsy". In Rho, Jong; Sankar, Raman (eds.). Epilepsy: Mechanisms, Models, and Translational Perspectives. CRC Press. p. 391. ISBN 978-1-4200-8560-0. Archived from the original on 13 November 2021. Retrieved 13 November 2021.
- 1 2 Eknoyan, Garabed (1997). "1. A history of diuretics". In Seldin, Donald W.; Giebisch, Gerhard H. (eds.). Diuretic Agents: Clinical Physiology and Pharmacology. San Diego: Academic Press. p. 24. ISBN 0-12-635690-4. Archived from the original on 13 November 2021. Retrieved 13 November 2021.
External links
| External sites: |
|
|---|---|
| Identifiers: |