Mozavaptan
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
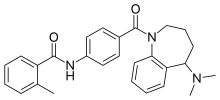
| Formula | C27H29N3O2 |
| Molar mass | 427.548 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Mozavaptan (INN) is a vasopressin receptor antagonist marketed by Otsuka. In Japan, it was approved in October 2006 for hyponatremia (low blood sodium levels) caused by syndrome of inappropriate antidiuretic hormone (SIADH) due to ADH producing tumors.
References
- Spreitzer H (November 20, 2006). "Neue Wirkstoffe - Conivaptan". Österreichische Apothekerzeitung (in German) (24/2006).
- "Conivaptan hydrochloride". Molecule of the Month. Prous Science. November 2006. Archived from the original on 2012-02-13.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.