Amiloride
 | |
| Names | |
|---|---|
| Trade names | Midamor, others |
| Other names | MK-870 |
IUPAC name
| |
| Clinical data | |
| Drug class | Potassium-sparing diuretic[1] |
| Main uses | High blood pressure, swelling due to heart failure or cirrhosis of the liver[1][2] |
| Side effects | High blood potassium, vomiting, loss of appetite, rash, headache[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| Onset of action | 2 hours (peak at 6–10 hours, duration ~24 hours) |
| Defined daily dose | 10 mg[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Readily absorbed, 15–25% |
| Protein binding | ~23% |
| Metabolism | Nil |
| Elimination half-life | 6 to 9 hours |
| Excretion | urine (20–50%), feces (40%) |
| Chemical and physical data | |
| Formula | C6H8ClN7O |
| Molar mass | 229.63 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 240.5 to 241.5 °C (464.9 to 466.7 °F) |
SMILES
| |
InChI
| |
Amiloride, sold under the trade name Midamor among others, is a medication typically used with other medications to treat high blood pressure or swelling due to heart failure or cirrhosis of the liver.[1][2] Amiloride is often used with a thiazide or other loop diuretic.[2] It is taken by mouth.[1] Onset of action is about two hours and it lasts for about a day.[2]
Common side effects include high blood potassium, vomiting, loss of appetite, rash, and headache.[1] The risk of high blood potassium is greater in those with kidney problems, diabetes, and those who are older.[1] Amiloride is in the potassium-sparing diuretic family of medications.[1] It works by increasing the amount of sodium and decreasing the amount of potassium released by the distal tubule of the kidney.[2]
Amiloride was developed in 1967.[4] It is on the World Health Organization's List of Essential Medicines.[5] In the United States, the wholesale price of a month of medication is about US$20.10.[6] In the United Kingdom, a month of medication costs the NHS about £24.[7]
Medical uses
Amiloride may be used in combination with a thiazide diuretic for treatment of high blood pressure or (less commonly) in combination with a loop diuretic for treatment of heart failure. The potassium-sparing effects of amiloride offset the low blood potassium (hypokalemia) that is often induced by thiazides or loop diuretics, which is of particular importance in people for whom maintaining a normal level of potassium is critically important.[8] For example, people that are taking Digitalis (i.e. digoxin) are at higher risk for changes in heart rhythm if their potassium levels get too high.[8] The 2017 clinical practice guidelines of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines list amiloride as a "secondary" oral antihypertensive, with minimal efficacy.[9] For people with resistant hypertension, already taking a thiazide diuretic, an angiotensin converting enzyme inhibitor (ACE-i) or an angiotensin II receptor blocker (ARB), and a calcium channel blocker, the addition of amiloride (or spironolactone) was better at reducing blood pressure than adding a beta-blocker (bisoprolol) or an alpha-1 blocker (doxazosin).[10] When combined with hydrochlorothiazide, the addition of amiloride had positive effects on blood pressure and blood sugar tolerance.[11] Amiloride may therefore be useful for preventing the metabolic side effects of thiazide diuretics, allowing for the use of higher thiazide doses (in line with how they were originally studied).[12]
Amiloride is the treatment of choice for Liddle phenotype,[13] which is characterized by high blood pressure, low blood potassium, and metabolic alkalosis in conjunction with a low plasma renin activity and a low aldosterone. Some people with the Liddle phenotype have Liddle syndrome, which involves a genetic mutation resulting in upregulation of the epithelial sodium channel (ENaC), located in the apical membrane of polarized epithelial cells in the late distal tubule and collecting duct of the kidney.[14] Because Liddle phenotype usually involves an upregulation of ENaC channels, leading to retention of sodium and water and to hypokalemia, amiloride is useful as an ENaC channel inhibitor due to its promotion of sodium excretion and its potassium-sparing effects, restoring potassium to normal levels.[15]
Amiloride can be used as a monotherapy (single-drug therapy) or an adjunctive therapy alongside other diuretics (e.g. hydrochlorothiazide, furosemide) for the treatment of ascites and edema (swelling) due to cirrhosis of the liver.[8] The 2012 clinical practice guidelines by the American Association for the Study of Liver Diseases (AASLD) states that amiloride can be used to treat ascites in place of spironolactone if it isn't tolerated (e.g. due to the side effect of gynecomastia), though amiloride isn't a preferred drug due to cost and lack of efficacy.[16]
Specific populations
Diabetics
People with diabetes are at higher risk for kidney problems, which increases their risk for hyperkalemia (high blood potassium). The use of amiloride in people with diabetes requires careful potassium and kidney function monitoring to prevent toxicity. Amiloride must be discontinued for at least 3 days prior to glucose tolerance testing, due to the risk for fatal hyperkalemia.[8]
Poor kidney function
People with poor kidney function (e.g. blood urea nitrogen >30 mg/dL, or serum creatinine >1.5 mg/dL) are at high risk for hyperkalemia.[8]
Lactation
There is no data on the use of amiloride in women that are breastfeeding. While diuretics can make lactation difficult, it is unlikely that amiloride would induce this effect in the absence of other diuretics.[17]
Pregnancy
Data from the use of amiloride in animals suggests that it does not pose a risk to the developing fetus. However, when used in combination with the drug acetazolamide during the process of organ formation, amiloride increases the risk for kidney and ureter abnormalities. Limited human data from use during pregnancy suggests an association with a specific congenital penis abnormality if taken during the first trimester, as well as a risk for mild intrauterine growth restriction if taken throughout pregnancy.[18]
Dosage
The defined daily dose is 10 mg (by mouth).[3]
Contraindications
Amiloride is contraindicated in people with kidney problems (e.g. anuria, acute or chronic kidney disease, or diabetic nephropathy), elevated blood potassium (≥5.5 mEq/L), or people that are hypersensitive to amiloride or any ingredients within the specific formulation. Use is also contraindicated in people that are already taking potassium-sparing drugs (e.g. spironolactone and triamterene) or whom are taking potassium supplements (e.g. potassium chloride) in most circumstances.[1]
Side effects
Amiloride is generally well tolerated.[19] Common adverse effects to the use of amiloride include elevated blood potassium, mild skin rashes, headaches, and gastrointestinal side effects (nausea, vomiting, diarrhea, decreased appetite, flatulence, and abdominal pain).[1] Mild symptoms of high blood potassium concentrations include unusual skin sensations, muscle weakness, or fatigue, but more severe symptoms such as flaccid paralysis of the limbs, slow heart rate, and even shock can occur.[1]
Overdose
There exists no overdose data on amiloride in humans, though it is expected than an overdose would produce effects consistent with its therapeutic effects; e.g. dehydration due to over-diuresis, and electrolyte disturbances related to hyperkalemia. It is unknown if amiloride can be dialyzed off, and no specific antidote against it exists. Treatment is generally supportive, though hyperkalemia can be treated.[19]
Interactions
Amiloride may have important drug-drug interactions when combined with other medications that also increase potassium levels in the blood, leading to hyperkalemia.[20] For example, the combination of amiloride with angiotensin-converting enzyme (ACE) inhibitors like lisinopril, or angiotensin II receptor type 1 (AT1) antagonists like losartan, may lead to high levels of potassium in the blood, requiring frequent monitoring.[20]
Pharmacology
Mechanism of action
Diuresis
Amiloride works by directly blocking the epithelial sodium channel (ENaC) with an IC50 around 0.1 μM, indicating potent blockade.[21] Antagonism of ENaC thereby inhibits sodium reabsorption in the late distal convoluted tubules, connecting tubules, and collecting ducts in the nephron.[22] This promotes the loss of sodium and water from the body, and reduces potassium excretion. The drug is often used in conjunction with a thiazide diuretic to counteract with a potassium-losing effect. Due to its potassium-sparing capacities, hyperkalemia (elevated potassium concentration in the blood) can occur. The risk of developing hyperkalemia is increased in patients who are also taking ACE inhibitors, angiotensin II receptor antagonists, other potassium-sparing diuretics, or any potassium-containing supplements.
Miscellaneous
A fraction of the effects of amiloride is inhibition of cyclic GMP-gated cation channels in the inner medullary collecting duct.[23]
Amiloride has a second action on the heart, blocking Na+/H+ exchangers sodium–hydrogen antiporter 1 or NHE-1. This minimizes re-perfusion injury in ischemic attacks.
Amiloride also blocks the Na+/H+ antiporter on the apical surface of the proximal tubule cells in the nephron, abolishing more than 80% of the action of angiotensin II on the secretion of hydrogen ions in proximal tubule cells.[24] Note that amiloride is not an angiotensin II receptor blocker (like losartan, for example). The Na-H transporter is also found in the Jejunum of the small intestine, as a result, amiloride also blocks the reabsoprtion of Na, and thereby water in the intestines.[25]
Amiloride is considered to be a reversible, pan-acid-sensing ion channel (ASIC) inhibitor that prevents the transient flow of ions but not the sustained flow of ions. ASICs are members of the ENaC family of protein channels, and are found in the nervous system, the cardiovascular system, the gastrointestinal system, and the skin. Broadly, ASICs are involved in harm detection, chemosensation (pH changes specifically), and touch.[26]
Pharmacokinetics
Absorption
Amiloride has an oral bioavailability of 50%, meaning that about 50% of an oral dose is absorbed into the blood stream. Coadministration with food reduces the amount of amiloride that is absorbed by the body by about 30%, though it does not affect the rate of absorption. However, taking amiloride with food helps to reduce the incidence of its gastrointestinal side effects. After being taken, amiloride's diuretic effect occurs within 2 hours, with peak diuresis within 6–10 hours. The diuretic effects of amiloride persist for about 24 hours after administration.[1]
Distribution
Amiloride cross the placenta and distributes into breast milk in vivo.[1]
Metabolism
Amiloride is not metabolized by the liver.[1] In comparison, the ENaC inhibitor triamterene is metabolized by the liver.[27]
Excretion
About 50% of amiloride is excreted unchanged by the kidneys, while around 40% is excreted in the feces (likely drug that wasn't absorbed). The half-life of amiloride in humans is between 6 and 9 hours, which may be prolonged in people with poor kidney function.[1]
Pharmacogenomics
A single nucleotide polymorphism (SNP) in the protein NEDD4L may impact how amiloride affects a person's blood pressure in people with high blood pressure.[28]
Chemistry

Amiloride's chemical structure is composed of a substituted pyrazine ring structure with a carbonylguanidinium substituent.[29] Amiloride's pKa is 8.67, which is due to the guanidinium group.[29] In high pH (alkaline, low hydrogen concentration) environments, the guanidinium group is deprotonated and the compound is rendered neutral, depleting its activity on sodium channels.[29] Amiloride, as a pure substance, is highly fluorescent, with excitation wavelengths at 215, 288, and 360 nm, emitting light at 420 nm.[30]

History
Amiloride was first synthesized and discovered by the Merck Sharp and Dohme Research Laboratories in the late 1960s.[29] The drug was discovered as part of a screening process of chemicals that reversed the effects of mineralocorticoids in vivo.[29] Amiloride was the only drug in the screen that was capable of causing the excretion of sodium (natriuresis) without a concomitant urinary excretion of potassium (kaliuresis).[29] Thousands of amiloride analogues have been studied since its initial discovery, which have been used to study the effects of sodium transporters.[29]
Amiloride was approved by the U.S. Food and Drug Administration (FDA) on October 5, 1981.[31]
Society and culture
It is on the World Health Organization's List of Essential Medicines.[5] In the United States the wholesale price of a month's supply at the usual daily dose of the medication is about US$20.10.[6] In the United Kingdom a month of medication costs the NHS about £24.[7]
Amiloride is on the World Anti-Doping Agency's list of banned substances, as it is considered a masking agent.[32] Diuretics like amiloride act as masking agents by reducing the concentration of other doping agents due to promoting diuresis, increasing the total volume of the urine.[27] The list includes other potassium-sparing diuretics, such as triamterene and spironolactone.[32] In 2008, amiloride and the potassium-sparing diuretic triamterene were found in 3% of positive diuretic doping samples.[27]
Formulations
- Amiloride hydrochloride
- Midamor (U.S.)
- Co-amilozide (amiloride hydrochloride with hydrochlorothiazide)
- Co-amilofruse (amiloride hydrochloride with furosemide)
- Amiloride hydrochloride with cyclopenthiazide
- Amiloride hydrochloride with bumetanide
Research
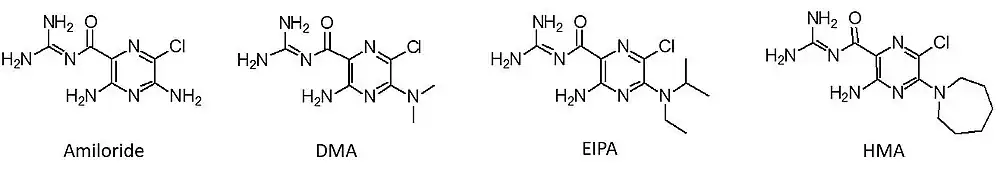
Amiloride is an inhibitor of NHE-1, which helps to maintain normal pH within cells. Cancer cells in leukemia, a type of blood cancer, have higher pH compared to normal cells. Amiloride affects the splicing and regulation of multiple genes involved in cancer, though they do not appear to be directly related to its effects on pH. Amiloride has been tested in vitro as an adjunct to the anticancer drug imatinib, which appeared to show a synergistic effect. Modified versions of amiloride, known as 5'-(N,N-dimethyl)-amiloride (DMA), 5-N-ethyl-N-isopropyl amiloride (EIPA), and 5-(N,N-hexamethylene)-amiloride (HMA), are being studied for the treatment of leukemia.[33]
Cystic fibrosis is a genetic disorder due to a mutation in the CFTR gene, which encodes for the CFTR chloride channel.[21] There is evidence that suggests that the molecular target of amiloride, ENaC, is also implicated in cystic fibrosis due to its effects on mucus in the lungs.[21] Aerosolized formulations of amiloride have been tested in clinical trials, though long-term clinical trials have failed to show much utility.[21] Due to its short duration of action, it was thought that longer-acting ENaC inhibitors may prove more effective.[34] However, longer-acting ENaC inhibitors (i.e. benzamil) have also failed clinical trials, despite an improvement in both the solubility and potency of the drugs.[21] A third generation amiloride analogue (N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N'-4-[4-(2,3-dihydroxypropoxy)phenyl]butyl-guanidine methanesulfonate,[35] research name "552-02"), with better pharmacokinetic properties, is being studied.[21]
![Side-by-side comparison of the chemical structures of amiloride and one of its analogues, research name 552-02 (N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N'-4-[4-(2,3-dihydroxypropoxy)phenyl]butyl-guanidine methanesulfonate).](../I/Amiloride55202.jpg.webp)
Pain induced by exposure to acid is attenuated by amiloride in human trials, which may indicate a role for amiloride in the treatment of pain in the future.[21]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 "Amiloride Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 27 December 2016. Retrieved 8 December 2016.
- 1 2 3 4 5 World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 328, 330. hdl:10665/44053. ISBN 9789241547659.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 26 October 2020. Retrieved 16 September 2020.
- ↑ Progress in Drug Research/Fortschritte der Arzneimittelforschung/Progrés des recherches pharmaceutiques. Birkhäuser. 2013. p. 210. ISBN 9783034870948. Archived from the original on 2016-12-28.
- 1 2 World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "NADAC as of 2016-12-07 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 21 December 2016. Retrieved 28 December 2016.
- 1 2 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 90. ISBN 9780857111562.
- 1 2 3 4 5 "MIDAMOR Product Monograph" (PDF). AA Pharma Inc. August 25, 2010. Archived (PDF) from the original on 28 April 2021. Retrieved 7 June 2018.
- ↑ Whelton, Paul K.; Carey, Robert M.; Aronow, Wilbert S.; Casey, Donald E.; Collins, Karen J.; Dennison Himmelfarb, Cheryl; DePalma, Sondra M.; Gidding, Samuel; Jamerson, Kenneth A.; Jones, Daniel W.; MacLaughlin, Eric J.; Muntner, Paul; Ovbiagele, Bruce; Smith, Sidney C.; Spencer, Crystal C.; Stafford, Randall S.; Taler, Sandra J.; Thomas, Randal J.; Williams, Kim A.; Williamson, Jeff D.; Wright, Jackson T. (June 2018). "2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines". Hypertension. 71 (6): e13–e115. doi:10.1161/HYP.0000000000000065. PMID 29133356.
- ↑ Williams, Bryan; MacDonald, Thomas M (2018). "Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies". Lancet Diabetes & Endocrinology. 6 (6): 464–475. doi:10.1016/S2213-8587(18)30071-8. PMC 5966620. PMID 29655877.
- ↑ Bavry, Anthony. "Prevention And Treatment of Hypertension With Algorithm based therapY-3 - American College of Cardiology". American College of Cardiology. American College of Cardiology Foundation. Archived from the original on 12 June 2018. Retrieved 9 June 2018.
- ↑ O'Riordan, Michael. "PATHWAY3: Amiloride-HCTZ Lowers BP With Neutral Effect on Glucose, Potassium". www.medscape.com. WebMD LLC. Archived from the original on 11 September 2017. Retrieved 9 June 2018.
- ↑ Spence JD (May 2017). "Rational Medical Therapy Is the Key to Effective Cardiovascular Disease Prevention". Can J Cardiol. 33 (5): 626–634. doi:10.1016/j.cjca.2017.01.003. PMID 28449833.
- ↑ Kellenberger S, Schild L (2015). "International Union of Basic and Clinical Pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel". Pharmacol. Rev. 67 (1): 1–35. doi:10.1124/pr.114.009225. PMID 25287517.
- ↑ Tetti, M; Monticone, S; Burrello, J; Matarazzo, P; Veglio, F; Pasini, B; Jeunemaitre, X; Mulatero, P (11 March 2018). "Liddle Syndrome: Review of the Literature and Description of a New Case". International Journal of Molecular Sciences. 19 (3): 812. doi:10.3390/ijms19030812. PMC 5877673. PMID 29534496.
- ↑ Runyon, Bruce (2012). "Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012" (PDF). American Association for the Study of Liver Disease. Archived from the original (PDF) on 12 June 2018. Retrieved 8 June 2018.
- ↑ "LACTMED: AMILORIDE". TOXNET. U.S. National Library of Medicine. Archived from the original on 16 February 2018. Retrieved 7 June 2018.
- ↑ "SafeFetus Drug Search". SafeFetus.com. SafeFetus.com. Archived from the original on 12 June 2018. Retrieved 8 June 2018.
- 1 2 "Approval Package for NDA 18-200/S-024". Center for Drug Evaluation and Research.
{{cite web}}: Missing or empty|url=(help) - 1 2 "Medicines and Hyperkalaemia". Medsafe. New Zealand Ministry of Health. Archived from the original on 28 January 2018. Retrieved 13 April 2019.
- 1 2 3 4 5 6 7 Qadri, Yawar J.; Rooj, Arun K.; Fuller, Catherine M. (April 2012). "ENaCs and ASICs as therapeutic targets". American Journal of Physiology. Cell Physiology. 302 (7): C943–C965. doi:10.1152/ajpcell.00019.2012. PMC 3330738. PMID 22277752.
- ↑ Loffing, Johannes; Kaissling, Brigitte (2003). "Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human". Am J Physiol Renal Physiol. 284 (4): F628–F643. doi:10.1152/ajprenal.00217.2002. PMID 12620920.
- ↑ Walter F. Boron (2005). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. ISBN 978-1-4160-2328-9. page 875
- ↑ Cogan, M. G. (1990). "Angiotensin II: A powerful controller of sodium transport in the early proximal tubule". Hypertension. 15 (5): 451–458. doi:10.1161/01.HYP.15.5.451. PMID 2185149. Archived from the original on 2016-09-19. Retrieved 2014-03-02.
- ↑ Gurney, Michael A.; Laubitz, Daniel; Ghishan, Fayez K.; Kiela, Pawel R. (January 2017). "Pathophysiology of Intestinal Na+/H+ Exchange". Cellular and Molecular Gastroenterology and Hepatology. 3 (1): 27–40. doi:10.1016/j.jcmgh.2016.09.010. PMC 5235326. PMID 28090568.
- ↑ Cheng, YR; Jiang, BY; Chen, CC (24 May 2018). "Acid-sensing ion channels: dual function proteins for chemo-sensing and mechano-sensing". Journal of Biomedical Science. 25 (1): 46. doi:10.1186/s12929-018-0448-y. PMC 5966886. PMID 29793480.
- 1 2 3 Cadwallader, AB; de la Torre, X; Tieri, A; Botrè, F (September 2010). "The abuse of diuretics as performance-enhancing drugs and masking agents in sport doping: pharmacology, toxicology and analysis". British Journal of Pharmacology. 161 (1): 1–16. doi:10.1111/j.1476-5381.2010.00789.x. PMC 2962812. PMID 20718736.
- ↑ "Amiloride - Variant Annotation". PharmGKB. PharmGKB. Archived from the original on 12 June 2018. Retrieved 8 June 2018.
- 1 2 3 4 5 6 7 Palmer L.G.; Kleyman T.R. (1995). "Potassium-Retaining Diuretics: Amiloride". In Greger, Rainer F.; Knauf, H.; Mutschler, E. (eds.). Diuretics. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 363–394. ISBN 978-3-642-79565-7.
- ↑ Sunkara, Prasad, ed. (2017). "11. Sodium Flux and Cancer Chemotherapy". Novel Approaches to Cancer Chemotherapy. Elsevier. p. 363. ISBN 9781483272177.
- ↑ "amiloride". drugcentral.org. Division of Translational Informatics at University of New Mexico. Archived from the original on 12 June 2018. Retrieved 8 June 2018.
- 1 2 "S5. Diuretics and masking agents - WADA". World Anti-Doping Agency. January 2016. Archived from the original on 27 September 2016. Retrieved 1 September 2016.
- ↑ Mihaila, Romeo Gabriel (3 December 2015). "A minireview on NHE1 inhibitors. A rediscovered hope in oncohematology". Biomedical Papers. 159 (4): 519–526. doi:10.5507/bp.2015.060. PMID 26725705.
- ↑ Rodgers, H. C.; Knox, A. J. (1 June 2001). "Pharmacological treatment of the biochemical defect in cystic fibrosis airways". European Respiratory Journal. 17 (6): 1314–1321. doi:10.1183/09031936.01.00086201. ISSN 0903-1936. PMID 11491179.
- ↑ Hirsh, A. J.; Zhang, J.; Zamurs, A.; Fleegle, J.; Thelin, W. R.; Caldwell, R. A.; Sabater, J. R.; Abraham, W. M.; Donowitz, M.; Cha, B.; Johnson, K. B.; St. George, J. A.; Johnson, M. R.; Boucher, R. C. (17 January 2008). "Pharmacological Properties of N-(3,5-Diamino-6-chloropyrazine-2-carbonyl)-N'-4-[4-(2,3-dihydroxypropoxy)phenyl]butyl-guanidine Methanesulfonate (552-02), a Novel Epithelial Sodium Channel Blocker with Potential Clinical Efficacy for Cystic Fibrosis Lung Disease". Journal of Pharmacology and Experimental Therapeutics. 325 (1): 77–88. doi:10.1124/jpet.107.130443. PMID 18218832. S2CID 40732094.
External links
| External sites: |
|
|---|---|
| Identifiers: |