Merotocin
 | |
| Clinical data | |
|---|---|
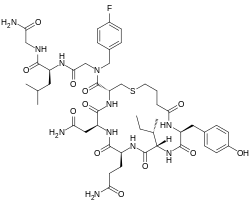
| Other names | N-(4-Sulfanylbutanoyl)-L-tyrosyl-L-isoleucyl-L-glutaminyl-L-asparaginyl-L-cysteinyl-N-[(4-fluorophenyl)methyl]glycyl-L-leucylglycinamide cyclic (1-5)-thioether |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C48H68FN11O12S |
| Molar mass | 1042.20 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Merotocin (INN) (developmental code name FE-202767), also known as carba-1-(4-FBzlGly7)dOT, is a peptidic agonist of the oxytocin receptor that was derived from oxytocin.[1][2][3] It is under development by Ferring Pharmaceuticals for the treatment of preterm mothers with lactation failure requiring lactation support, and is in phase II clinical trials for this indication.[3] Merotocin is potent (EC50 < 0.1 nM) and highly selective (>1000-fold over the related vasopressin receptors).
See also
References
- ↑ Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G (2012). "Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics". Journal of Neuroendocrinology. 24 (4): 609–28. doi:10.1111/j.1365-2826.2012.02303.x. PMC 3490377. PMID 22375852.
- ↑ Yang Y, Li H, Ward R, Gao L, Wei JF, Xu TR (2014). "Novel oxytocin receptor agonists and antagonists: a patent review (2002 - 2013)". Expert Opinion on Therapeutic Patents. 24 (1): 29–46. doi:10.1517/13543776.2014.845168. PMID 24094047. S2CID 10584554.
- 1 2 Wiśniewski K, Alagarsamy S, Galyean R, Tariga H, Thompson D, Ly B, Wiśniewska H, Qi S, Croston G, Laporte R, Rivière PJ, Schteingart CD (2014). "New, potent, and selective peptidic oxytocin receptor agonists". J. Med. Chem. 57 (12): 5306–17. doi:10.1021/jm500365s. PMID 24874785.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.