Carbetocin
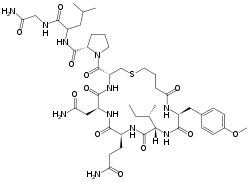 | |
| Names | |
|---|---|
| Trade names | Duratocin, Pabal, Lonactene, others |
| Other names | (2-O-Methyltyrosine)deamino-1-carbaoxytocin; Deamino-2-O-methyltyrosine-1-carbaoxytocin; 1-Butanoic acid-2-(O-methy-L-tyrosine)-1-carbaoxytocin; 1-butyric acid-2-[3-(4-methoxyphenyl)-L-alanine]oxytocin |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Intravenous, intramuscular |
| Defined daily dose | 0.1 mg[1] |
| External links | |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 80% (IM) |
| Elimination half-life | 85–100 minutes[2] |
| Chemical and physical data | |
| Formula | C45H69N11O12S |
| Molar mass | 988.17 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Carbetocin, sold under the brand names Pabal among others, is a medication used to prevent excessive bleeding after childbirth, particularly following Cesarean section.[3] It appears to work as well as oxytocin.[4] Due to it being less economical than other options, use is not recommended by NHS Scotland.[3] It is given by injection into a vein or muscle.[4]
Side effects differ little from that of no treatment or placebo.[4] Use is not recommended in people with epilepsy or eclampsia.[3] Carbetocin is manufactured long acting form of oxytocin.[4] It works by activating the oxytocin receptor which causes the uterus to contract.[5][4]
Carbetocin was first described in 1974.[6] It was approved for medical use in Canada and the United Kingdom in 1997.[4] It is on the World Health Organization's List of Essential Medicines.[7] In the United Kingdom it costs the NHS £18 a dose as of 2018.[3] Globally prices range from £8 to £40.[4] It is not available in the United States or Japan.[8][4]
Medical uses
Carbetocin has been approved for use immediately following an elective Cesarean section when a local or spinal anesthesia has been used.[9] Since the uterus cannot contract on its own following incision during a Cesarean section[10], exogenous administration of oxytocin or an analog is necessary to restore uterine tone and prevent hemorrhage.[11]Safety of carbetocin following vaginal births and emergency Cesarean sections has not been established, though a Cochrane review suggested efficacy following vaginal births[12]. The review indicated that a 100 ug dose following vaginal delivery caused contractions and little or no adverse side effects.[12]
Carbetocin has also been shown to increase uterine involution (the return of the uterus to its contracted state after the birth of the baby) in humans, horses and cows.[13][14]
Comparisons
In 2018, a meta-analysis found carbetocin, to be as good as oxytocin for reduction of postpartum hemorrhage after vaginal delivery, though there is monetary difference between both[15] It is hoped that this will make oxytocic hemorrhage control more widely available and less expensive, which will be particularly useful in regions of developing countries where the cold chain (in drug transport and storage) is unreliable because of power outages or equipment problems.[16][17]
Due to carbetocin's considerably longer half-life, its effects are longer lasting than other oxytocin homologs such as oxytocin or barusiban.[18] A single carbetocin dose compared to a placebo or an eight-hour intravenous drip of oxytocin in a randomized blind study, necessitated less additional oxytocin therapy following a Cesarean section. Oxytocin receptor antagonists, such as barusiban or atosiban have the opposite effect of depressing oxytocin receptor activity and can be used to stop premature labor and uterine contractions.[18]
Dosage
The defined daily dose is 0.1 mg (by injection).[1]
Side effects
Ten to forty percent of people will experience nausea, vomiting, abdominal pain, itching skin, increased body temperature, trembling and weakness. A small percent of people may experience back and chest pain, dizziness, anemia, chills and sweating, metallic taste, tachycardia and respiratory distress.[19][20][8] Contraindications for the use of carbetocin include inappropriate timing during labor and delivery (such as before parturition or to induce labor) or allergic reactions to carbetocin or other oxytocin homologues.[19] Additionally, carbetocin should not be used if a person has high blood pressure or cardiovascular problems. Overdosage or repeated use of carbetocin, particularly if used during pregnancy, could cause hyper-excitation of the oxytocin receptors resulting in excessive and prolonged stimulation of uterine contractions, increasing risk of uterine rupture, placental abruption, fetal respiratory distress and postpartum hemorrhage.[19]
Interactions
Oxytocin analogs often bind with much lower affinity to vasopressin receptors V1, in the uterine lining, and V2, in the kidneys [21].Carbetocin may work synergistically with drugs such as ergometrine that ripen the cervix[22], concurrent use of carbetocin and misoprostol is not recommended
Mechanism of action
Carbetocin works as an oxytocic, antihemorrhagic and uterotonic drug in the peripheral nervous system.[23] The most common causes of postpartum hemorrhage are lack of tone in the uterus from overstretching [24]
Carbetocin functions as an agonist at peripheral oxytocin receptors, particularly in the myometrium, with lesser affinity for myoepithelial cells. Oxytocin receptors are G protein-coupled[25] and their mechanism of action involves second messengers and the production of inositol phosphates.[18] Carbetocin mimics this mechanism.[26] Binding for carbetocin and other oxytocin agonists has been shown to be nonselective at the extracellular N-terminus and loops E2 and E3.[18] While the oxytocin receptor shows equal affinity for oxytocin and carbetocin, the biological effect of carbetocin is almost 50% that of endogenous or exogenous oxytocin.[26][18] Carbetocin has a much longer lasting effect than oxytocin, necessitating only a single dose. Carbetocin inhibits endogenous oxytocin release, interrupting the uterine feedback loop with the hypothalamus and decreasing both central and peripheral release of oxytocin.[25]
During pregnancy, the synthesis of oxytocin receptors in the uterus greatly increases, reaching a peak during labor and delivery. Consequently, the administration of carbetocin or another oxytocin analog during or immediately following birth will have increased uterotonic and contractile effect.[27] Carbetocin also functions to thicken the blood, further preventing post-partum hemorrhage.[20] Carbetocin should be used to induce or augment labor with extreme caution since it could cause cardiac problems for the infant.[28]
Pharmacokinetics
Carbetocin is to be used in the hospital by prescription only. It can be administered intravenously or intramuscularly. In both cases, the recommended dose for an average adult female is 100 ug. Contractile effects of the uterus are apparent within two minutes and can be observed for approximately one hour,[19] though maximum binding occurs about 30 minutes after intramuscular injection. Administration is performed immediately following parturition to minimize risk of postpartum hemorrhage by inducing uterine contractions, increasing muscle tone and thickening the blood. If further uterine stimulation is needed, treatment with other forms of oxytocic uterotonic drugs should be used.[19]
Endogenous and synthetic oxytocin has a half-life of approximately 3.5 minutes.[26] Carbetocin, in comparison, has a much longer half-life ranging from 85–100 minutes.[26] The bioavailable dose is around 80%.[29] The elimination half-life following intravenous administration is around 40 minutes, though the elimination mechanism is not entirely known.[19] Studies have shown that elimination is only minimally renal (0.7%), but may occur at least partially through enzymatic degradation of peptides, primarily on the C-terminal end.[26] Both elimination and volume of distribution are not dose dependent.[19]
Society and culture
Legal approval
Carbetocin has been approved for use under the following three brand names in 23 countries, not including the United States: Duratocin (Argentina, Australia, Bahrain, Canada, China, Hong Kong, Italy, Malaysia, Singapore, New Zealand), Lonactene (Mexico), and Pabal (Austria, Belgium, Switzerland, Germany, Estonia, France, UK, Hungary, Lithuania, Luxembourg). Duratocin has also been approved for veterinary use in Poland, Germany, Italy, Belgium, Luxembourg, France and the Netherlands.[20]
Brand names
Duratocin, Pabal, Lonactene, are among the brand names[4]
Cost
In the United Kingdom it costs the NHS £17.64 a dose as of 2018.[3] Globally prices range from £8 to £40.[4]
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 27 November 2020. Retrieved 10 September 2020.
- ↑ Idan Shalev; Richard Paul Ebstein (11 February 2015). Social Hormones and Human Behavior: What Do We Know and Where Do We Go from Here. Frontiers Media SA. pp. 51–. ISBN 978-2-88919-407-0. Archived from the original on 14 March 2017. Retrieved 27 September 2016.
- 1 2 3 4 5 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 804. ISBN 9780857113382.
- 1 2 3 4 5 6 7 8 9 10 "PROPOSAL FOR INCLUSION OF CARBETOCIN IN THE WHO LIST OF ESSENTIAL MEDICINES" (PDF). WHO. Archived (PDF) from the original on 31 January 2020. Retrieved 12 November 2019.
- ↑ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 65–. ISBN 978-94-011-4439-1.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 214–. ISBN 978-1-4757-2085-3.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 "Carbetocin Drug Information, Professional". Drugs.com. Archived from the original on 28 November 2020. Retrieved 26 December 2020.
- ↑ Heesen, M.; Carvalho, B.; Carvalho, J. C. A.; Duvekot, J. J.; Dyer, R. A.; Lucas, D. N.; McDonnell, N.; Orbach‐Zinger, S.; Kinsella, S. M. (2019). "International consensus statement on the use of uterotonic agents during caesarean section". Anaesthesia. 74 (10): 1305–1319. doi:10.1111/anae.14757. ISSN 1365-2044. Archived from the original on 15 August 2021. Retrieved 19 December 2020.
- ↑ The Science of Pregnancy: The Complete Illustrated Guide From Conception to Birth. Penguin. 2019. p. 232. ISBN 978-1-4654-9921-9. Archived from the original on 28 August 2021. Retrieved 30 December 2020.
- ↑ Yamaguchi, Eduardo Tsuyoshi; Siaulys, Mônica Maria; Torres, Marcelo Luis Abramides (July 2016). "Oxytocin in cesarean-sections. What's new?". Brazilian Journal of Anesthesiology (English Edition). 66 (4): 402–407. doi:10.1016/j.bjane.2014.11.015. ISSN 0104-0014. Archived from the original on 29 November 2020. Retrieved 30 December 2020.
- 1 2 Su, Lin-Lin; Chong, Yap-Seng; Samuel, Miny (18 April 2012). "Carbetocin for preventing postpartum haemorrhage". The Cochrane Database of Systematic Reviews (4): CD005457. doi:10.1002/14651858.CD005457.pub4. ISSN 1469-493X. Archived from the original on 28 August 2021. Retrieved 30 December 2020.
- ↑ Bajcsy, AC; Szenci, O; van der Weijden, GC; Doornenbal, A; Maassen, F; Bartyik, J; Taverne, MA (20 January 2006). "The effect of a single oxytocin or carbetocin treatment on uterine contractility in early postpartum dairy cows". Theriogenology. 65 (2): 400–14. doi:10.1016/j.theriogenology.2005.05.040. PMID 15993938.
- ↑ Schramme, AR; Pinto, CR; Davis, J; Whisnant, CS; Whitacre, MD (November 2008). "Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, following intravenous administration in horses". Equine Veterinary Journal. 40 (7): 658–61. doi:10.2746/042516408X334343. PMID 19165935.
- ↑ Voon, Hian Yan; Suharjono, Haris Njoo; Shafie, Asrul Akmal; Bujang, Mohamad Adam (June 2018). "Carbetocin versus oxytocin for the prevention of postpartum hemorrhage: A meta-analysis of randomized controlled trials in cesarean deliveries". Taiwanese Journal of Obstetrics & Gynecology. 57 (3): 332–339. doi:10.1016/j.tjog.2018.04.002. ISSN 1875-6263. Archived from the original on 28 August 2021. Retrieved 30 December 2020.
- ↑ Malm M; et al. (2018), "Development and stability of a heat-stable formulation of carbetocin for the prevention of postpartum haemorrhage for use in low and middle-income countries", J Pept Sci, 24 (6): e3082, doi:10.1002/psc.3082, PMC 6001700, PMID 29700898.
- ↑ Mundasad, Smitha (28 June 2018). "Revamped drug could save lives of many new mothers: WHO". BBC News Online. Archived from the original on 7 July 2018. Retrieved 28 June 2018.
- 1 2 3 4 5 Gimpl, G; Postina, R; Fahrenholz, F; Reinheimer, T (7 March 2005). "Binding domains of the oxytocin receptor for the selective oxytocin receptor antagonist barusiban in comparison to the agonists oxytocin and carbetocin". European Journal of Pharmacology. 510 (1–2): 9–16. doi:10.1016/j.ejphar.2005.01.010. PMID 15740719.
- 1 2 3 4 5 6 7 "Product Information - Duratocin". healthlinks.net. Archived from the original on 15 November 2011. Retrieved 5 June 2012.
- 1 2 3 "Carbetocin". drugs.com. Archived from the original on 3 March 2016. Retrieved 5 June 2012.
- ↑ Vrachnis, Nikolaos; Malamas, Fotodotis M.; Sifakis, Stavros; Deligeoroglou, Efthymios; Iliodromiti, Zoe (6 December 2011). "The Oxytocin-Oxytocin Receptor System and Its Antagonists as Tocolytic Agents". International Journal of Endocrinology. Archived from the original on 2 June 2018. Retrieved 31 December 2020.
- ↑ "WHO recommendations Uterotonics for the prevention of postpartum haemorrhage" (PDF). World Health Orhanization. Who.int. Archived (PDF) from the original on 7 July 2021. Retrieved 31 December 2020.
- ↑ Sachdeva, Sakshi (1 March 2017). "Peptides as 'Drugs': The Journey so Far". International Journal of Peptide Research and Therapeutics. 23 (1): 49–60. doi:10.1007/s10989-016-9534-8. ISSN 1573-3904. Archived from the original on 10 June 2018. Retrieved 23 December 2020.
- ↑ Anderson, Janice M.; Etches, Duncan (15 March 2007). "Prevention and management of postpartum hemorrhage". American Family Physician. 75 (6): 875–882. ISSN 0002-838X. Archived from the original on 20 April 2021. Retrieved 30 December 2020.
- 1 2 Gimpl, G; Fahrenholz, F (April 2001). "The oxytocin receptor system: structure, function, and regulation". Physiological Reviews. 81 (2): 629–83. doi:10.1152/physrev.2001.81.2.629. PMID 11274341. S2CID 13265083.
- 1 2 3 4 5 Engstrøm, T; Barth, T; Melin, P; Vilhardt, H (21 August 1998). "Oxytocin receptor binding and uterotonic activity of carbetocin and its metabolites following enzymatic degradation". European Journal of Pharmacology. 355 (2–3): 203–10. doi:10.1016/S0014-2999(98)00513-5. PMID 9760035.
- ↑ Chao, Yi-Sheng; McCormack, Suzanne (2019). "Carbetocin for the Prevention of Post-Partum Hemorrhage: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines". Canadian Agency for Drugs and Technologies in Health. Archived from the original on 28 August 2021. Retrieved 30 December 2020.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Visser, Gerard H. A.; Kayser, Angela (2015). "2.14 - Uterine contraction agents, tocolytics, vaginal therapeutics and local contraceptives". Drugs During Pregnancy and Lactation (Third Edition). Academic Press. pp. 401–412. ISBN 978-0-12-408078-2. Archived from the original on 28 August 2021. Retrieved 21 December 2020.
- ↑ Padubidri, V. (2018). Textbook of Obstetrics. Wolters kluwer india Pvt Ltd. p. 418. ISBN 978-93-87506-15-2. Archived from the original on 28 August 2021. Retrieved 22 December 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |