PF-184563
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | 34% |
| Protein binding | 69% |
| Elimination half-life | 1.8h |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
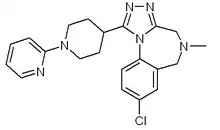
| Formula | C21H23ClN6 |
| Molar mass | 394.91 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
PF-184563 is a potent, selective non-peptidic antagonist of the V1a receptor.[1] The compound was discovered by Pfizer in its Sandwich, Kent research center, as a potential treatment for dysmenorrhoea, an indication for which V1a antagonists have shown efficacy.[2]
References
- ↑ Johnson PS, Ryckmans T, Bryans J, Beal DM, Dack KN, Feeder N, Harrison A, Lewis M, Mason HJ, Mills J, Newman J, Pasquinet C, Rawson DJ, Roberts LR, Russell R, Spark D, Stobie A, Underwood TJ, Ward R, Wheeler S (October 2011). "Discovery of PF-184563, a potent and selective V1a antagonist for the treatment of dysmenorrhoea. The influence of compound flexibility on microsomal stability". Bioorg. Med. Chem. Lett. 21 (19): 5684–5687. doi:10.1016/j.bmcl.2011.08.038. PMID 21885275.
- ↑ Brouard R, Bossmar T, Fournie-Lloret D, Chassard D, Akerlund M (2000). "Effect of SR49059, an orally active V1a vasopressin receptor antagonist, in the prevention of dysmenorrhoea". Br. J. Obstet. Gynaecol. 107 (5): 614–619. doi:10.1111/j.1471-0528.2000.tb13302.x. PMID 10826575. S2CID 19439985.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.