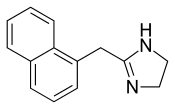
Naphazoline
 | |
 | |
| Names | |
|---|---|
| Trade names | Naphcon-a, Clear Eyes Red Relief, Visine A, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Alpha adrenergic agonist[1] |
| Main uses | Stuffy nose, eye redness[1] |
| Side effects | Burning, blurry vision[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Nasal spray, eye drops |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Chemical and physical data | |
| Formula | C14H14N2 |
| Molar mass | 210.274 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Naphazoline, sold under many brand names, is a medication used to treat a stuffy nose or eye redness due to minor irritation.[1] It is available as a nasal spray or eye drops.[1]
Common side effects include blurry vision and stinging.[1] Other side effects may include recurrence of stuffiness following stopping use, headache, palpitations, and nervousness.[1] Safety in pregnancy is unclear.[1] It works by activating alpha adrenergic receptor which cases small arteries to narrow.[1]
Naphazoline was patented in 1934 and came into medical use in 1942.[2] It is available as a generic medication and over the counter.[1] In the United States 15 ml of solution costs about 14 USD.[3]
Medical use
Dosage
It is used at a dose of 1 to 2 eye drops every 3 to 4 hours.[1]
Side effects
A few warnings and contraindications that apply to all naphazoline-containing substances intended for medicinal use are:
- Hypersensitivity to naphazoline
- Patients taking MAO inhibitors can experience a severe hypertensive crisis if given a sympathomimetic drug such as naphazoline HCl
- Use in infants and children can result in central nervous system depression, leading to coma and marked reduction in body temperature
- Should be used with caution in patients with severe cardiovascular disease including cardiac arrhythmia and in patients with diabetes, especially those with a tendency toward diabetic ketoacidosis
- Drug interactions can occur with anaesthetics that sensitize the myocardium to sympathomimetics (e.g. cyclopropane or halothane cautiously)
- Exercise caution when applying prior to use of phenylephrine.
- Extended use may cause rhinitis medicamentosa, a condition of rebound nasal congestion.
A possible association with stroke has been suggested.[4]
Chemistry
The non-hydrochloride form of Naphazoline has the molecular formula C14H14N2 and a molar mass of 210.28 g/mol. The HCl salt form has a molar mass of 246.73 g/mol.
Society and culture
Brand names
It is an active ingredient in several over-the-counter formulations including Rohto, Eucool, Clear Eyes and Naphcon eye drops.[5]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Naphazoline Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 11 November 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 552. ISBN 9783527607495. Archived from the original on 2016-12-29. Retrieved 2020-10-19.
- ↑ "Naphazoline ophthalmic Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 11 November 2021.
- ↑ Zavala JA, Pereira ER, Zétola VH, Teive HA, Nóvak EM, Werneck LC (September 2004). "Hemorrhagic stroke after naphazoline exposition: case report". Arquivos de Neuro-Psiquiatria. 62 (3B): 889–91. doi:10.1590/S0004-282X2004000500030. PMID 15476091.
- ↑ Green SM (2008). "Ophthalmology: Naphazoline". Tarascon Pocket Pharmacopoeia 2009. Jones and Bartlett. ISBN 978-0-7637-6572-9.
External links
| Identifiers: |
|---|