Buflomedil
 | |
| Names | |
|---|---|
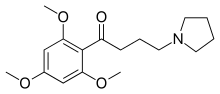
| Preferred IUPAC name
4-(Pyrrolidin-1-yl)-1-(2,4,6-trimethoxyphenyl)butan-1-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.054.393 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C17H25NO4 |
| Molar mass | 307.38 g/mol |
| Pharmacology | |
| C04AX20 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Buflomedil, sold under the brand name Loftyl, is a vasoactive drug used to treat claudication or the symptoms of peripheral arterial disease. It is currently not approved by the Food and Drug Administration (FDA) for use in the United States.
Toxicity
This drug has been suspended from marketing in the European Union, because of concerns about severe neurological and cardiac toxicity.[1][2] In its press release dated 17 November 2011 EMA suggested that doctors "should stop using buflomedil and consider alternative treatment options". The European Commission advised all member states to revoke marketing authorisation.[3]
Various adverse effects have been reported to the FDA.[4]
References
- ↑ Medscape: http://www.medscape.com/viewarticle/753768
- ↑ EMA: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/public_health_alerts/2011/11/human_pha_detail_000045.jsp&mid=WC0b01ac058001d126
- ↑ https://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Pharmakovigilanz/Risikoinformationen/RisikoBewVerf/a-f/buflomedil_durchf_beschluss.pdf?__blob=publicationFile&v=3
- ↑ http://www.drugcite.com/?q=BUFLOMEDIL&s=&a=
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.