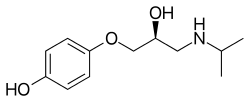
Prenalterol
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.246 |
| Chemical and physical data | |
| Formula | C12H19NO3 |
| Molar mass | 225.288 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Prenalterol is a cardiac stimulant which acts as a β1 adrenoreceptor agonist.[1]
Synthesis
Stereospecific
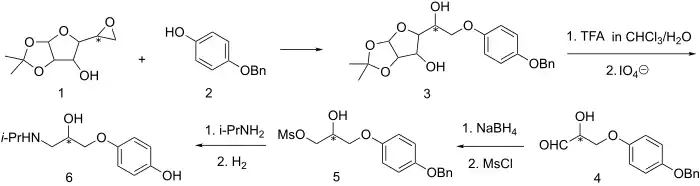
Prenalterol exhibits adrenergic agonist activity in spite of an interposed oxymethylene group. The stereospecific synthesis devised for this molecule relies on the fact that the side chain is very similar in oxidation state to that of a sugar.
Condensation of monobenzone (2) with the epoxide derived from α-D-glucofuranose[5] affords the glycosylated derivative (3). Hydrolytic removal of the acetonide protecting groups[6] followed by cleavage of the sugar with periodate gives aldehyde (4). This is reduced to the glycol by means of NaBH4 and the terminal alcohol is converted to the mesylate (5). Displacement of the leaving group with isopropylamine followed by hydrogenolytic removal of the O-benzyl ether affords the β1-adrenergic selective adrenergic agonist prenalterol (6).
Racemic
Prepns of the racemic mixture: NL 6409883 corresp to H. Köppe et al., U.S. Patent 3,637,852 (1965, 1972 both to Boehringer Ingelheim); NL 301580 corresp to A. F. Crowther, L. H. Smith, U.S. Patent 3,501,769 (1965, 1970 both to ICI);[7]
Further reading
- Acta Medica Scandinavica. 211. 1982. doi:10.1111/joim.1982.211.issue-s659.
{{cite journal}}: Missing or empty|title=(help)
See also
References
- ↑ Hadfield SE, Slee SJ, Snow HM (1989). "The cardiovascular pharmacology of xamoterol, cicloprolol, prenalterol and pindolol in the anaesthetised dog". Br J Clin Pharmacol. 28 Suppl 1 (Suppl 1): 78S–81S. doi:10.1111/j.1365-2125.1989.tb03580.x. PMC 1379883. PMID 2572262.
- ↑ DE 2503968, Jaeggi, Knut A.; Schröter, Herbert & Ostermayer, Franz, "Optisch aktive Derivate des 1-Phenoxy-2-hydroxy-3-aminopropan und Verfahren zu ihrer Herstellung [Optically active derivatives of 1-phenoxy-2-hydroxy-3-aminopropane and the process for their production]", published 1975-08-14, assigned to Ciba-Geigy AG
- ↑ K. A. Jaeggi, H. Schroeter, and F. Ostermayer, Chem. Abstr. 84, 5322 (1976).
- ↑ corresp to U.S. Patent 3,978,041 and U.S. Patent 4,049,797 (1975, 1976, 1977, all to Ciba-Geigy).
- ↑ http://www.chemspider.com/Chemical-Structure.9312824.html
- ↑ Liu, Z; Hu, B. H.; Messersmith, P. B. (2010). "Acetonide Protection of Dopamine for the Synthesis of Highly Pure N-docosahexaenoyldopamine". Tetrahedron Letters. 51 (18): 2403–2405. doi:10.1016/j.tetlet.2010.02.089. PMC 2882309. PMID 20543896.
- ↑ Crowther, Albert F.; Gilman, D. J.; McLoughlin, B. J.; Smith, Leslie Harold; Turner, R. W.; Wood, T. M. (1969). ".beta.-Adrenergic blocking agents. V. 1-Amino-3-(substituted phenoxy)-2-propanols". Journal of Medicinal Chemistry. 12 (4): 638–42. doi:10.1021/jm00304a018. PMID 5793156.