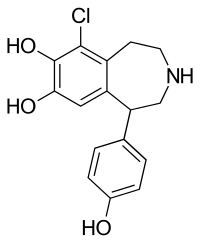
Fenoldopam
 | |
 | |
| Names | |
|---|---|
| Trade names | Corlopam |
| Other names | Fenoldopam mesylate |
IUPAC name
| |
| Clinical data | |
| Drug class | Dopamine D1 receptor agonist[1] |
| Main uses | Hypertensive emergencies[2] |
| Side effects | Headache, flushing, nausea, low blood pressure[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | IV |
| Onset of action | Within 10 min[3] |
| Duration of action | An hour[3] |
| Typical dose | 0.01 to 1.6 mcg/kg/min[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Liver (CYP not involved) |
| Elimination half-life | 5 minutes |
| Excretion | Kidney (90%) and fecal (10%) |
| Chemical and physical data | |
| Formula | C16H16ClNO3 |
| Molar mass | 305.76 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Fenoldopam, sold under the brand name Corlopam, is a medication used to treat high blood pressure.[2] Specifically it is used short term for hypertensive emergencies.[2] It is given by continuous injection into a vein.[2] Effects begin within 10 minutes and last for up to an hour.[3]
Common side effects include headache, flushing, nausea, and low blood pressure.[2] Other side effects may include anaphylaxis, palpitations, low potassium, and increased eye pressure.[2] Risk in pregnancy is unclear.[4] It is a dopamine D1 receptor agonist which results in blood vessel dilation.[2][1] It is a benzazepine derivative.[2]
Fenoldopam was approved for medical use in the United States in 1997.[2] It is available as a generic medication.[3] In the United States it costs about 400 USD per 10 mg as of 2021.[5]
Medical uses
Fenoldopam is used as an antihypertensive agent postoperatively, and also intravenously (IV) to treat a hypertensive crisis.[6] Since fenoldopam is an intravenous agent with minimal adrenergic effects that improves kidney perfusion, in theory it could be beneficial in people with hypertensive and chronic kidney disease.[3]
Its effectiveness is similar to nitroprusside without the concerns regarding cyanide toxicity.[2] It however is much more expensive.[7]
Dosage
In adults for severe blood pressure it is started at 0.01 to 0.3 mcg/kg/minute then increase by 0.05 to 0.1 mcg/kg/minute at 15 minute intervals until desired blood pressure is reached or a max of 1.6 mcg/kg/minute is reached.[3]
Side effects
Side effects include headache, flushing, nausea, hypotension, reflex tachycardia, and increased intraocular pressure.[6][8]
Fenoldopam contains sodium metabisulfite, a sulfite that may rarely cause allergic-type reactions including anaphylactic symptoms and asthma in susceptible people. Fenoldopam mesylate administration should be undertaken with caution to patients with glaucoma or raised intraocular pressure as fenoldopam raises intraocular pressure.[8] Concomitant use of fenoldopam with a beta-blocker should be avoided if possible, as unexpected hypotension can result from beta-blocker inhibition of sympathetic-mediated reflex tachycardia in response to fenoldopam.[8]
Pharmacology
Fenoldopam causes arterial/arteriolar vasodilation leading to a decrease in blood pressure by activating peripheral D1 receptors.[9] It decreases afterload and also promotes sodium excretion via specific dopamine receptors along the nephron. The renal effect of fenoldopam and dopamine may involve physiological antagonism of the renin-angiotensin system in the kidney.[10] In contrast to dopamine, fenoldopam is a selective D1 receptor agonist with no effect on beta adrenoceptors, although there is evidence that it may have some alpha-1 [11] and alpha-2 adrenoceptor antagonist activity.[9] D1 receptor stimulation activates adenylyl cyclase and raises intracellular cyclic AMP, resulting in vasodilation of most arterial beds, including renal, mesenteric, and coronary arteries.[12] to cause a reduction in systemic vascular resistance. Fenoldopam has a rapid onset of action (4 minutes) and short duration of action (< 10 minutes) and a linear dose–response relationship at usual clinical doses.[13]
References
- 1 2 "Drug Information Portal - U.S. National Library of Medicine - Quick Access to Quality Drug Information". druginfo.nlm.nih.gov. Archived from the original on 28 April 2021. Retrieved 10 December 2021.
- 1 2 3 4 5 6 7 8 9 10 11 "Fenoldopam Monograph for Professionals". Drugs.com. Archived from the original on 12 May 2021. Retrieved 10 December 2021.
- 1 2 3 4 5 6 7 Szymanski, Michael W.; Richards, John R. (2021), "Fenoldopam", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30252314, archived from the original on 2020-08-12, retrieved 2021-05-02
- ↑ "Fenoldopam (Corlopam) Use During Pregnancy". Drugs.com. Archived from the original on 4 December 2020. Retrieved 10 December 2021.
- ↑ "Corlopam Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 10 December 2021.
- 1 2 Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 9. ISBN 978-1-59541-101-3.
- ↑ Devlin, JW; Seta, ML; Kanji, S; Somerville, AL (May 2004). "Fenoldopam versus nitroprusside for the treatment of hypertensive emergency". The Annals of pharmacotherapy. 38 (5): 755–9. doi:10.1345/aph.1D363. PMID 15039472.
- 1 2 3 NDA 19-922/S-005 Archived 2021-04-03 at the Wayback Machine: Corlopam RA06497-R1-9/03 brand of Fenoldopam Mesylate Injection, USP
- 1 2 Nichols AJ, Ruffolo RR, Brooks DP (June 1990). "The pharmacology of fenoldopam". Am. J. Hypertens. 3 (6 Pt 2): 116S–119S. doi:10.1093/ajh/3.6.116s. PMID 1974439.
- ↑ Gildea JJ (January 2009). "Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance". Curr. Opin. Nephrol. Hypertens. 18 (1): 28–32. doi:10.1097/MNH.0b013e32831a9e0b. PMC 2847451. PMID 19077686.
- ↑ Martin SW, Broadley KJ (May 1995). "Renal vasodilatation by dopexamine and fenoldopam due to alpha 1-adrenoceptor blockade". Br. J. Pharmacol. 115 (2): 349–55. doi:10.1111/j.1476-5381.1995.tb15884.x. PMC 1908310. PMID 7670737.
- ↑ Hughes AD, Sever PS (1989). "Action of fenoldopam, a selective dopamine (DA1) receptor agonist, on isolated human arteries". Blood Vessels. 26 (2): 119–27. doi:10.1159/000158760. PMID 2474340.
- ↑ Epstein, Murray MD, "Diagnosis and Management of Hypertensive Emergencies," clinical Cornerstone. Hypertension Vol2. No 1.
External links
| Identifiers: |
|---|