Lenperone
 | |
| Clinical data | |
|---|---|
| Trade names | Elanone-V |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.166 |
| Chemical and physical data | |
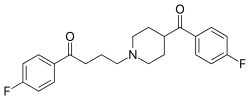
| Formula | C22H23F2NO2 |
| Molar mass | 371.428 g·mol−1 |
Lenperone (Elanone-V) is a typical antipsychotic of the butyrophenone chemical class.[1] It was first reported as an emetic in 1974,[2] and its use in treatment of acute schizophrenia was reported in 1975.[1] Related early antipsychotic agents include declenperone and milenperone.
Lenperone was never approved by the FDA for use in humans in the United States,[3] but prior to 1989 it was approved for use in veterinary medicine for sedation.[4][5][6]
See also
Chemically related drugs containing the same 4-(p-fluorobenzoyl)piperidine group:
- Altanserin
- Ketanserin
- Lidanserin
- Setoperone
References
- 1 2 Harris M (1975). "Treatment of Acute Schizophrenia with a New Butyrophenone-Lenperone". Journal of Clinical Pharmacology. 15 (2–3): 187–90. doi:10.1002/j.1552-4604.1975.tb02355.x. PMID 1091666. S2CID 28602974.
- ↑ FR 2227868, Ward, John Wesley & Leonard, Charles A., "Antiemetic compositions containing piperidine derivatives", published 1974-11-29
- ↑ Table 1. Marketed butyrophenones with approval status and indication in Miller AC, Khan AM, Castro Bigalli AA, Sewell KA, King AR, Ghadermarzi S, et al. (2019). "Neuroleptanalgesia for acute abdominal pain: a systematic review". Journal of Pain Research. 12: 787–801. doi:10.2147/JPR.S187798. PMC 6396833. PMID 30881092.
- ↑ Booth NJ (1982). "Psychotropic Agents". In Booth NH, McDonald LE (ed.). Veterinary Pharmacology and Therapeutics (5th ed.). Ames, Iowa: Iowa State University Press. pp. 321–345.
- ↑ Johnson SE, Zelner A, Sherding RG (April 1989). "Effect of lenperone hydrochloride on gastroesophageal sphincter pressure in healthy dogs". Canadian Journal of Veterinary Research. 53 (2): 248–50. PMC 1255555. PMID 2565757.
- ↑ FDA Veterinarian. U.S. Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Veterinary Medicine. 1988.
The firm requested withdrawal of approval because the products are no longer being marketed. Effective date: July 13, 1989
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Histamine receptor modulators | |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
See also: Receptor/signaling modulators • Monoamine metabolism modulators • Monoamine reuptake inhibitors | |||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.