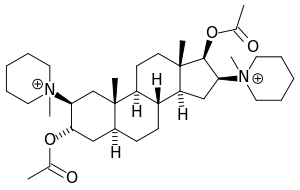
Pancuronium
 | |
 | |
| Names | |
|---|---|
| Other names | Pancuronium bromide |
IUPAC name
| |
| Clinical data | |
| Drug class | Neuromuscular blocking agent[1] |
| Main uses | Relax muscles during general anesthesia or during intubation and ventilation[2] |
| Side effects | Muscle weakness, anaphylaxis, increased heart rate[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Onset of action | 2 to 3 min[2] |
| Duration of action | Up to 100 min[2] |
| Typical dose | 0.04 to 0.1 mg/kg[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | NA |
| Protein binding | 77 to 91% |
| Metabolism | Liver |
| Elimination half-life | 1.5 to 2.7 hours |
| Excretion | Kidney and biliary |
| Chemical and physical data | |
| Formula | C35H60N2O4 |
| Molar mass | 572.875 g·mol−1 |
InChI
| |
Pancuronium, sold under the brand name Pavulon, is a medication used to relax muscles during general anesthesia, when a person is being ventilated, and to help with endotracheal intubation.[2] It is given by injection into a vein.[2] Onset is relatively slow at 2 to 3 minutes while effects can last for up to 100 minutes.[2]
Common side effects include muscle weakness.[2] Other side effects may include anaphylaxis and increased heart rate.[2] Use in pregnancy appears to be safe, but is not well studied.[3] Neostigmine or pyridostigmine may be used to reverse its affects.[1] It is a neuromuscular blocking agent.[1]
Pancuronium was first made in 1964 and approved for medical use in the United States in 1972.[2][1] It is available as a generic medication.[4] In the United Kingdom it costs the NHS about £5 per 4 mg as of 2021.[4] This amount is about 3 USD in the United States.[5] It has been used in euthanasia and during lethal injection.[6][7]
Medical uses
Pancuronium is used with general anesthesia in surgery for muscle relaxation and as an aid to intubation or ventilation. It does not have sedative or analgesic effects.
In Belgium and the Netherlands, pancuronium is recommended in the protocol for euthanasia. After administering sodium thiopental to induce coma, pancuronium is delivered in order to stop breathing.[7]
Dosage
The typical initial dose is 0.04 to 0.1 mg/kg with further doses of 0.01 mg/kg as needed.[2] May also be given as an infusion at 0.7 to 2 mcg/kg/min.[1] The dose is based on a persons ideal body weight.[4]
Side effects
Sideeffects include moderately raised heart rate and thereby arterial pressure and cardiac output, excessive salivation, apnea and respiratory depression, rashes, flushing, and sweating. The muscular relaxation can be dangerous in the seriously ill and it can accumulate leading to extended weakness. Pancuronium is not preferable in long-term use in ICU-ventilated patients.
Mechanism of action
Pancuronium is a typical non-depolarizing curare-mimetic muscle relaxant. It competitively inhibits the nicotinic acetylcholine receptor at the neuromuscular junction by blocking the binding of acetylcholine. It has slight vagolytic activity, causing an increase in heart rate, but no ganglioplegic (i.e., blocking ganglions) activity. It is a very potent muscle relaxant drug, with an ED95 (i.e., the dose that causes 95% depression of muscle twitch response) of only 60 µg/kg body weight. Onset of action is relatively slow compared to other similar drugs, in part due to its low dose: an intubating dose takes 3–6 minutes for full effect. Clinical effects (muscle activity lower than 25% of physiological) last for about 100 minutes. The time needed for full (over 90% muscle activity) recovery after single administration is about 120–180 minutes in healthy adults.
The effects of pancuronium can be at least partially reversed by anticholinesterasics, such as neostigmine, pyridostigmine, and edrophonium.
History
Pancuronium is designed to mimic the action of two molecules of acetylcholine with the quaternary nitrogen atoms spaced rigidly apart by the steroid rings at a distance of ten atoms (interonium distance). Decamethonium and suxamethonium also have this same interonium distance.
Society and culture
Lethal injection
Pancuronium is also used as one component of a lethal injection in administration of the death penalty in some parts of the United States.[6]
Controversy
Like all non-depolarising muscle relaxants, pancuronium has no effect on level of consciousness. Therefore, if the anaesthetic used is insufficient, the individual may be awake but unable to cry out or move due to the effect of the pancuronium. There have been several civil lawsuits alleging similar failures of adequate anaesthesia during general surgical procedures. These have been largely due to improper or insufficient dosages of anaesthetic in concert with normal dosages of muscle relaxants such as pancuronium.
In 2007, Michael Munro, a Scottish neonatologist at Aberdeen Maternity Hospital, was cleared of malpractice by the GMC Fitness to Practice panel after giving 23 times the standard dose of pancuronium to two dying neonates. Terminally ill, both dying babies were suffering from agonal gasping and violent body spasms, which was highly distressing for the parents to witness. Munro then administered pancuronium to the babies after advising the parents that this would ease their suffering and could also hasten death.[8][9] It is on record that neither of the children's parents were unhappy with Dr Munro's treatment of their babies.[10]
Amnesty International has objected to its use in lethal injections on the grounds that it "may mask the condemned prisoner's suffering during the execution,"[11] thereby leading observers to conclude that lethal injection is painless, or less cruel than other forms of execution.
Export limitations
The United Kingdom bans the export of pancuronium bromide to the United States due to its use in lethal injections, but not to Netherlands or Belgium.[12]
Uses in crime
Pancuronium was used in Efren Saldivar's killing spree.[13] It was also used by the Skin Hunters to kill patients in the Polish city of Łódź. Pavulon was also used by Richard Angelo in 1987 to kill at least 10 patients under his care at the Good Samaritan Hospital in New York.
References
- 1 2 3 4 5 Das GN, Sharma P, Maani CV (January 2021). "Pancuronium". StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. PMID 30855929. Archived from the original on 2021-01-04. Retrieved 2021-08-22.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Pancuronium Monograph for Professionals". Drugs.com. Archived from the original on 5 October 2021. Retrieved 26 October 2021.
- ↑ "Pancuronium (Pavulon) Use During Pregnancy". Drugs.com. Archived from the original on 30 November 2020. Retrieved 26 October 2021.
- 1 2 3 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1391. ISBN 978-0857114105.
- ↑ "Pancuronium Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 10 May 2019. Retrieved 26 October 2021.
- 1 2 "US court backs lethal injection". BBC News. 16 April 2008. Archived from the original on 20 April 2008. Retrieved 22 August 2021.
- 1 2 "Administration and Compounding Of Euthanasic Agents". The Hague: Royal Dutch Society for the Advancement of Pharmacy. Archived from the original on 7 June 2008. Retrieved 15 July 2008 – via ERGO!.
- ↑ "Baby doctor cleared of misconduct". BBC News. 11 July 2007. Archived from the original on 2007-10-08. Retrieved 2010-05-21.
- ↑ "Doctor cleared over baby deaths". The Guardian. 11 July 2007. Archived from the original on 2 October 2021. Retrieved 22 August 2021.
- ↑ "Doctor felt babies were suffering". BBC News. 9 July 2007. Archived from the original on 2007-09-14. Retrieved 2010-05-21.
- ↑ "UA 44/04 Death penalty". Amnesty International. 6 February 2004. Archived from the original on 17 May 2004.
- ↑ "Provisions supplementing "the torture Regulation"". Article 4A of Export Control Order 2008, UK Statutory Instruments 2008 No. 3231 PART 2 Article 9. UK Legislation. Archived from the original on 2021-10-01. Retrieved 2021-08-22.
- ↑ Ramsland K (9 April 2005). "Dark Rumors". Crimelibrary. Archived from the original on 9 April 2005.
External links
| Identifiers: |
|---|