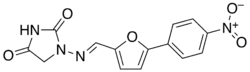
Dantrolene
 | |
 | |
| Names | |
|---|---|
| Trade names | Dantrium, Revonto, Ryanodex |
IUPAC name
| |
| Clinical data | |
| Main uses | Malignant hyperthermia, neuroleptic malignant syndrome, muscle spasticity[1][2] |
| Side effects | Sleepiness, dizziness, weakness, diarrhea[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | By mouth, intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682576 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 70% |
| Metabolism | Liver |
| Excretion | Biliary, kidney |
| Chemical and physical data | |
| Formula | C14H10N4O5 |
| Molar mass | 314.257 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dantrolene sodium, sold under the brand name Dantrium among others, is a medication used to treat malignant hyperthermia, neuroleptic malignant syndrome, and muscle spasticity.[1][2] This may include spasticity such as due to multiple sclerosis, cerebral palsy, or stroke.[2] Other possible uses include MDMA toxicity.[5] It may be used by injection into a vein or by mouth.[1]
Common side effects include sleepiness, dizziness, weakness, and diarrhea.[2] Intravenous use may also result in inflammation at the site.[2] Other side effects may include liver problems or palpitations.[1] It works by blocking Ca2+ ions release by skeletal muscles and thereby preventing their contraction.[1]
Dantrolene was first made in 1967 by Snyder.[5] It was approved for medical use in the United States in 1974.[2] It is available as a generic medication.[6] Hospitals are recommended to keep at least 36 vials totaling 720 mg on hand, enough for a 70-kg person.[7] This amount in the United Kingdom costs the NHS about £1,800 and in the United States about 3,000 USD as of 2020.[1][8]
Medical uses

It is used to treat malignant hyperthermia, neuroleptic malignant syndrome, and muscle spasticity.[1][2] This may include spasticity such as due to multiple sclerosis, cerebral palsy, or stroke.[2] Other possible uses include ecstasy toxicity.[5]
Dosage
For malignant hyperthermia the initial dose is generally 2 to 3 mg per kg.[1] Repeated doses of 1 mg per kg may be given.[1] The maximum dose is 10 mg per kg.[1]
Side effects
Central nervous system side effects are quite frequently noted and encompass speech and visual disturbances, mental depression and confusion, hallucinations, headache, insomnia and exacerbation or precipitation of seizures, and increased nervousness. Infrequent cases of respiratory depression or a feeling of suffocation have been observed. Dantrolene often causes sedation severe enough to incapacitate the patient to drive or operate machinery.
Gastrointestinal effects include bad taste, decreased appetite, nausea, vomiting, abdominal cramps, and diarrhea.
Liver side effects may be seen either as asymptomatic elevation of liver enzymes and/or bilirubin or, most severe, as fatal and nonfatal liver inflammation. The risk of liver inflammation is associated with the duration of treatment and the daily dose. In patients treated for hyperthermia, no liver toxicity has been observed so far. Patients on chronic dantrolene therapy should routinely have LFTs monitored.
Pleural effusion with inflammation of the fibrous sac around the heart (oral treatment only), rare cases of bone marrow damage, diffuse muscle pains, backache, dermatologic reactions, transient cardiovascular reactions, and crystals in the urine have additionally been seen. Muscle weakness may persist for several days following treatment.
Mutagenicity and carcinogenity
It is unclear whether dantrolene has carcinogenic effects.
Contraindications
Oral dantrolene cannot be used by:
- people with a pre-existing liver disease
- people with compromised lung function
- people with severe cardiovascular impairment
- people with a known hypersensitivity to dantrolene
- pediatric patients under five years of age
- people who need good muscular balance or strength to maintain an upright position, motoric function, or proper neuromuscular balance
If the indication is a medical emergency, such as malignant hyperthermia, the only significant contraindication is hypersensitivity.
Pregnancy and breastfeeding
If needed in pregnancy, adequate human studies are lacking, therefore the drug should be given in pregnant women only if clearly indicated. It may cause hypotonia in the newborn if given closely before delivery.[5]
Dantrolene should not be given to breastfeeding mothers. If a treatment is necessary, breastfeeding should be terminated.
Interactions
Dantrolene may interact with the following drugs:[9]
- Calcium channel blockers of the diltiazem/verapamil type: Intravenous treatment with dantrolene and concomitant calcium channel blocker treatment may lead to severe cardiovascular collapse, abnormal heart rhythms, myocardial depressions, and high blood potassium.
- Nondepolarizing neuromuscular blocking agents, such as vecuronium bromide: Neuromuscular blockade is potentiated.
- CNS depressants: Sedative action is potentiated. Benzodiazepines may also cause additive muscle weakness.
- Combined oral contraceptives and hormone replacement therapy with estrogens may enhance liver toxicity of dantrolene, particularly in women over 35 years of age.
Pharmacology
Dantrolene depresses excitation-contraction coupling in skeletal muscle by acting as a receptor antagonist to the ryanodine receptor, and decreasing free intracellular calcium concentration.[5]
Chemistry
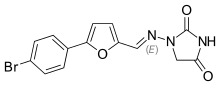
Chemically it is a hydantoin derivative, but does not exhibit antiepileptic activity like other hydantoin derivates such as phenytoin.[5]
The poor water solubility of dantrolene leads to certain difficulties in its use.[5][10] A more water-soluble analog of dantrolene, azumolene, is under development for similar indications.[10] Azumolene has a bromine residue instead of the nitro group found in dantrolene, and is 30 times more water-soluble.[5]
Synthesis
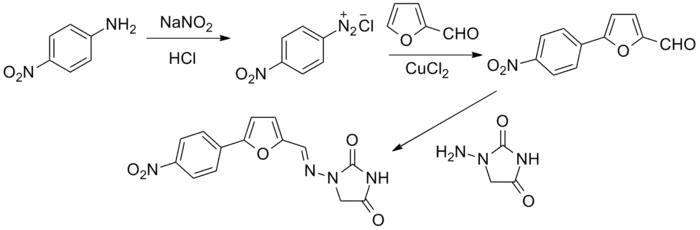
The original patent synthesis started with para-nitroaniline which undergoes diazotization followed by a copper(II) chloride catalyzed arylation with furfural (essentially a modified Meerwein arylation). This then reacts with 1-aminohydantoin to form the final product.
History
Dantrolene was first described in the scientific literature in 1967, as one of several hydantoin derivatives proposed as a new class of muscle relaxant.[12] Dantrolene underwent extensive further development, and its action on skeletal muscle was described in detail in 1973.[13]
Dantrolene was widely used in the management of spasticity[14] before its efficacy in treating malignant hyperthermia was discovered by South African anesthesiologist Gaisford Harrison and reported in a landmark 1975 article published in the British Journal of Anaesthesia.[15] Harrison experimentally induced malignant hyperthermia with halothane anesthesia in genetically susceptible pigs, and obtained an 87.5% survival rate, where seven of his eight experiments survived after intravenous administration of dantrolene. The efficacy of dantrolene in humans was later confirmed in a large, multicenter study published in 1982,[16] and confirmed epidemiologically in 1993.[17] Before dantrolene, the only available treatment for malignant hyperthermia was procaine, which was associated with a 60% mortality rate in animal models.[15]
References
- 1 2 3 4 5 6 7 8 9 10 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1417. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - 1 2 3 4 5 6 7 8 9 "Dantrolene Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 21 July 2021.
- 1 2 "Dantrolene Use During Pregnancy". Drugs.com. 9 December 2019. Archived from the original on 25 January 2021. Retrieved 6 July 2020.
- ↑ "Dantrium 25mg Capsules - Summary of Product Characteristics (SmPC)". (emc). 28 February 2020. Archived from the original on 7 July 2020. Retrieved 6 July 2020.
- 1 2 3 4 5 6 7 8 Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F (2004). "Dantrolene – a review of its pharmacology, therapeutic use and new developments". Anaesthesia. 59 (4): 364–73. doi:10.1111/j.1365-2044.2004.03658.x. PMID 15023108. S2CID 18537509.
- ↑ "Dantrolene Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 13 October 2016. Retrieved 21 July 2021.
- ↑ Yeung EY, Munroe J (2015). "Development of a malignant hyperthermia protocol". BMC Proceedings. 9 (Suppl1): A32. doi:10.1186/1753-6561-9-S1-A32. PMC 4306034.
- ↑ Ho, PT; Carvalho, B; Sun, EC; Macario, A; Riley, ET (August 2018). "Cost-benefit Analysis of Maintaining a Fully Stocked Malignant Hyperthermia Cart versus an Initial Dantrolene Treatment Dose for Maternity Units". Anesthesiology. 129 (2): 249–259. doi:10.1097/ALN.0000000000002231. PMID 29672336.
- ↑ "Dantrolene Drug Interactions". Epocrates Online. Epocrates. 2008. Archived from the original on 2012-02-17. Retrieved 2021-07-06. Retrieved on December 31, 2008.
- 1 2 Sudo RT, Carmo PL, Trachez MM, Zapata-Sudo G (March 2008). "Effects of azumolene on normal and malignant hyperthermia-susceptible skeletal muscle". Basic Clin Pharmacol Toxicol. 102 (3): 308–16. doi:10.1111/j.1742-7843.2007.00156.x. PMID 18047479.
- ↑ Snyder, H. R.; Davis, C. S.; Bickerton, R. K.; Halliday, R. P. (1967). "1-[5-Arylfurfurylidene)amino]hydantoins. A New Class of Muscle Relaxants". Journal of Medicinal Chemistry. 10 (5): 807–10. doi:10.1021/jm00317a011. PMID 6048486.
- ↑ Snyder HR, Davis CS, Bickerton RK, Halliday RP (September 1967). "1-[(5-arylfurfurylidene)amino]hydantoins. A new class of muscle relaxants". J Med Chem. 10 (5): 807–10. doi:10.1021/jm00317a011. PMID 6048486.
- ↑ Ellis KO, Castellion AW, Honkomp LJ, Wessels FL, Carpenter JE, Halliday RP (June 1973). "Dantrolene, a direct acting skeletal muscle relaxant". J Pharm Sci. 62 (6): 948–51. doi:10.1002/jps.2600620619. PMID 4712630.
- ↑ Pinder, RM; Brogden, RN; Speight, TM; Avery, GS (January 1977). "Dantrolene sodium: a review of its pharmacological properties and therapeutic efficacy in spasticity". Drugs. 13 (1): 3–23. doi:10.2165/00003495-197713010-00002. PMID 318989. S2CID 7936488.
- 1 2 Harrison GG (January 1975). "Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium". Br J Anaesth. 47 (1): 62–5. doi:10.1093/bja/47.1.62. PMID 1148076. S2CID 21991166. A reprint of the article, which became a "Citation Classic", is available in Harrison GG (October 1998). "Control of the malignant hyperpyrexic syndrome in MHS swine by dantrolene sodium. 1975". Br J Anaesth. 81 (4): 626–9, discussion 625. doi:10.1093/bja/81.4.626. PMID 9924249.
- ↑ Kolb ME, Horne ML, Martz R (April 1982). "Dantrolene in human malignant hyperthermia". Anesthesiology. 56 (4): 254–62. doi:10.1097/00000542-198204000-00005. PMID 7039419. S2CID 72069279.
- ↑ Strazis KP, Fox AW (March 1993). "Malignant hyperthermia: review of published cases". Anesth Analg. 77 (3): 297–304. doi:10.1213/00000539-199377020-00014. PMID 8346828.
External links
| Identifiers: |
|---|
- "Dantrolene". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-04-13. Retrieved 2021-07-06.
- "Dantrolene sodium". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-07-07. Retrieved 2021-07-06.