Cerebral palsy
| Cerebral palsy | |
|---|---|
 | |
| A child with cerebral palsy being assessed by a physician | |
| Specialty | Pediatrics, neurology, physiatry |
| Symptoms | Poor coordination, stiff muscles, weak muscles, tremors[1] |
| Complications | Seizures, intellectual disability[1] |
| Usual onset | Early childhood[1] |
| Duration | Lifelong[1] |
| Causes | Often unknown[1] |
| Risk factors | Preterm birth, being a twin, certain infections during pregnancy, difficult delivery[1] |
| Diagnostic method | Based on child's development[1] |
| Treatment | Physical therapy, occupational therapy, speech therapy, external braces, orthopedic surgery[1] |
| Medication | Diazepam, baclofen, botulinum toxin[1] |
| Frequency | 2.1 per 1,000[2] |
Cerebral palsy (CP) is a group of permanent movement disorders that appear in early childhood.[1] Signs and symptoms vary among people and over time.[1][3] Often, symptoms include poor coordination, stiff muscles, weak muscles, and tremors.[1] There may be problems with sensation, vision, hearing, swallowing, and speaking.[1] Often, babies with cerebral palsy do not roll over, sit, crawl or walk as early as other children of their age.[1] Other symptoms include seizures and problems with thinking or reasoning, which each occur in about one third of people with CP.[1] While symptoms may get more noticeable over the first few years of life, underlying problems do not worsen over time.[1]
Cerebral palsy is caused by abnormal development or damage to the parts of the brain that control movement, balance, and posture.[1][4] Most often, the problems occur during pregnancy; however, they may also occur during childbirth or shortly after birth.[1] Often, the cause is unknown.[1] Risk factors include preterm birth, being a twin, certain infections during pregnancy such as toxoplasmosis or rubella, exposure to methylmercury during pregnancy, a difficult delivery, and head trauma during the first few years of life, among others.[1] About 2% of cases are believed to be due to an inherited genetic cause.[5] A number of sub-types are classified based on the specific problems present.[1] For example, those with stiff muscles have spastic cerebral palsy, those with poor coordination have ataxic cerebral palsy and those with writhing movements have athetoid cerebral palsy.[1] Diagnosis is based on the child's development over time.[1] Blood tests and medical imaging may be used to rule out other possible causes.[1]
CP is partly preventable through immunization of the mother and efforts to prevent head injuries in children such as through improved safety.[1] There is no known cure for CP; however, supportive treatments, medications and surgery may help many individuals.[1] This may include physical therapy, occupational therapy and speech therapy.[1] Medications such as diazepam, baclofen and botulinum toxin may help relax stiff muscles.[1] Surgery may include lengthening muscles and cutting overly active nerves.[1] Often, external braces and other assistive technology are helpful.[1] Some affected children can achieve near normal adult lives with appropriate treatment.[1] While alternative medicines are frequently used, there is no evidence to support their use.[1]
Cerebral palsy is the most common movement disorder in children.[6] It occurs in about 2.1 per 1,000 live births.[2] Cerebral palsy has been documented throughout history, with the first known descriptions occurring in the work of Hippocrates in the 5th century BCE.[7] Extensive study of the condition began in the 19th century by William John Little, after whom spastic diplegia was called "Little's disease".[7] William Osler first named it "cerebral palsy" from the German zerebrale Kinderlähmung (cerebral child-paralysis).[8] A number of potential treatments are being examined, including stem cell therapy.[1] However, more research is required to determine if it is effective and safe.[1]
Signs and symptoms
Cerebral palsy is defined as "a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain."[9] While movement problems are the central feature of CP, difficulties with thinking, learning, feeling, communication and behavior often co-occur,[9] with 28% having epilepsy, 58% having difficulties with communication, at least 42% having problems with their vision, and 23–56% having learning disabilities.[10] Muscle contractions in people with cerebral palsy are commonly thought to arise from overactivation.[11]
Cerebral palsy is characterized by abnormal muscle tone, reflexes, or motor development and coordination. The neurological lesion is primary and permanent while orthopedic manifestations are secondary and progressive. In cerebral palsy unequal growth between muscle-tendon units and bone eventually leads to bone and joint deformities. At first deformities are dynamic. Over time, deformities tend to become static, and joint contractures develop. Deformities in general and static deformities in specific (joint contractures) cause increasing gait difficulties in the form of tip-toeing gait, due to tightness of the Achilles tendon, and scissoring gait, due to tightness of the hip adductors. These gait patterns are among the most common gait abnormalities in children with cerebral palsy. However, orthopaedic manifestations of cerebral palsy are diverse.[12][13] The effects of cerebral palsy fall on a continuum of motor dysfunction, which may range from slight clumsiness at the mild end of the spectrum to impairments so severe that they render coordinated movement virtually impossible at the other end of the spectrum. Although most people with CP have problems with increased muscle tone, some have normal or low muscle tone. High muscle tone can either be due to spasticity or dystonia.[14]
Babies born with severe cerebral palsy often have an irregular posture; their bodies may be either very floppy or very stiff. Birth defects, such as spinal curvature, a small jawbone, or a small head sometimes occur along with CP. Symptoms may appear or change as a child gets older. Babies born with cerebral palsy do not immediately present with symptoms.[15] Classically, CP becomes evident when the baby reaches the developmental stage at 6 to 9 months and is starting to mobilise, where preferential use of limbs, asymmetry, or gross motor developmental delay is seen.[13]
Drooling is common among children with cerebral palsy, which can have a variety of impacts including social rejection, impaired speaking, damage to clothing and books, and mouth infections.[16] It can additionally cause choking.[17]
An average of 55.5% of people with cerebral palsy experience lower urinary tract symptoms, more commonly excessive storage issues than voiding issues. Those with voiding issues and pelvic floor overactivity can deteriorate as adults and experience upper urinary tract dysfunction.[18]
Children with CP may also have sensory processing issues.[19] Adults with cerebral palsy have a higher risk of respiratory failure.[20]
Skeleton
For bones to attain their normal shape and size, they require the stresses from normal musculature.[21] People with cerebral palsy are at risk of low bone mineral density.[22] The shafts of the bones are often thin (gracile),[21] and become thinner during growth. When compared to these thin shafts (diaphyses), the centres (metaphyses) often appear quite enlarged (ballooning). Due to more than normal joint compression caused by muscular imbalances, articular cartilage may atrophy,[23]: 46 leading to narrowed joint spaces. Depending on the degree of spasticity, a person with CP may exhibit a variety of angular joint deformities. Because vertebral bodies need vertical gravitational loading forces to develop properly, spasticity and an abnormal gait can hinder proper or full bone and skeletal development. People with CP tend to be shorter in height than the average person because their bones are not allowed to grow to their full potential. Sometimes bones grow to different lengths, so the person may have one leg longer than the other.
Children with CP are prone to low trauma fractures, particularly children with higher GMFCS levels who cannot walk. This further affects a child's mobility, strength, experience of pain, and can lead to missed schooling or child abuse suspicions.[21] These children generally have fractures in the legs, whereas non-affected children mostly fracture their arms in the context of sporting activities.[24]
Hip dislocation and ankle equinus or planter flexion deformity are the two most common deformities among children with cerebral palsy. Additionally, flexion deformity of the hip and knee can occur. Besides, torsional deformities of long bones such as the femur and tibia are encountered among others.[12][25] Children may develop scoliosis before the age of 10 – estimated prevalence of scoliosis in children with CP is between 21% and 64%.[26] Higher levels of impairment on the GMFCS are associated with scoliosis and hip dislocation.[12][27] Scoliosis can be corrected with surgery, but CP makes surgical complications more likely, even with improved techniques.[26] Hip migration can be managed by soft tissue procedures such as adductor musculature release. Advanced degrees of hip migration or dislocation can be managed by more extensive procedures such as femoral and pelvic corrective osteotomies. Both soft tissue and bony procedures aim at prevention of hip dislocation in the early phases or aim at hip containment and restoration of anatomy in the late phases of disease.[12] Equinus deformity is managed by conservative methods especially when dynamic. If fixed/static deformity ensues surgery may become mandatory.[25]
Growth spurts during puberty can make walking more difficult.[28]
Eating
Due to sensory and motor impairments, those with CP may have difficulty preparing food, holding utensils, or chewing and swallowing. An infant with CP may not be able to suck, swallow or chew.[29] Gastro-oesophageal reflux is common in children with CP.[17] Children with CP may have too little or too much sensitivity around and in the mouth.[29] Poor balance when sitting, lack of control of the head, mouth and trunk, not being able to bend the hips enough to allow the arms to stretch forward to reach and grasp food or utensils, and lack of hand-eye coordination can make self-feeding difficult.[30] Feeding difficulties are related to higher GMFCS levels.[17] Dental problems can also contribute to difficulties with eating.[30] Pneumonia is also common where eating difficulties exist, caused by undetected aspiration of food or liquids.[17] Fine finger dexterity, like that needed for picking up a utensil, is more frequently impaired than gross manual dexterity, like that needed for spooning food onto a plate.[31] Grip strength impairments are less common.[31]
Children with severe cerebral palsy, particularly with oropharyngeal issues, are at risk of undernutrition.[32] Triceps skin fold tests have been found to be a very reliable indicator of malnutrition in children with cerebral palsy.[30]
Language
Speech and language disorders are common in people with cerebral palsy. The incidence of dysarthria is estimated to range from 31% to 88%,[33] and around a quarter of people with CP are non-verbal.[34] Speech problems are associated with poor respiratory control, laryngeal and velopharyngeal dysfunction, and oral articulation disorders that are due to restricted movement in the oral-facial muscles. There are three major types of dysarthria in cerebral palsy: spastic, dyskinetic (athetosis), and ataxic.[35]
Early use of augmentative and alternative communication systems may assist the child in developing spoken language skills.[34] Overall language delay is associated with problems of cognition, deafness, and learned helplessness.[36] Children with cerebral palsy are at risk of learned helplessness and becoming passive communicators, initiating little communication.[36] Early intervention with this clientele, and their parents, often targets situations in which children communicate with others so that they learn that they can control people and objects in their environment through this communication, including making choices, decisions, and mistakes.[36]
Pain and sleep
Pain is common and may result from the inherent deficits associated with the condition, along with the numerous procedures children typically face.[37] When children with cerebral palsy are in pain, they experience worse muscle spasms.[38] Pain is associated with tight or shortened muscles, abnormal posture, stiff joints, unsuitable orthosis, etc. Hip migration or dislocation is a recognizable source of pain in CP children and especially in the adolescent population. Nevertheless, the adequate scoring and scaling of pain in CP children remains challenging.[12] Pain in CP has a number of different causes, and different pains respond to different treatments.[39]
There is also a high likelihood of chronic sleep disorders secondary to both physical and environmental factors.[40] Children with cerebral palsy have significantly higher rates of sleep disturbance than typically developing children.[41] Babies with cerebral palsy who have stiffness issues might cry more and be harder to put to sleep than non-disabled babies, or "floppy" babies might be lethargic.[42] Chronic pain is under-recognized in children with cerebral palsy,[43] even though 3 out of 4 children with cerebral palsy experience pain.[44]
Associated disorders
Associated disorders include intellectual disabilities, seizures, muscle contractures, abnormal gait, osteoporosis, communication disorders, malnutrition, sleep disorders, and mental health disorders, such as depression and anxiety.[45] In addition to these, functional gastrointestinal abnormalities contributing to bowel obstruction, vomiting, and constipation may also arise. Adults with cerebral palsy may have ischemic heart disease, cerebrovascular disease, cancer, and trauma more often.[46] Obesity in people with cerebral palsy or a more severe Gross Motor Function Classification System assessment in particular are considered risk factors for multimorbidity.[47] Other medical issues can be mistaken for being symptoms of cerebral palsy, and so may not be treated correctly.[48]
Related conditions can include apraxia, dysarthria or other communication disorders, sensory impairments, urinary incontinence, fecal incontinence, or behavioural disorders.
Seizure management is more difficult in people with CP as seizures often last longer.[49] Epilepsy and asthma are common co-occurring diseases in adults with CP.[50] The associated disorders that co-occur with cerebral palsy may be more disabling than the motor function problems.[17]
Causes
Cerebral palsy is due to abnormal development or damage occurring to the developing brain.[51] This damage can occur during pregnancy, delivery, the first month of life, or less commonly in early childhood.[51] Structural problems in the brain are seen in 80% of cases, most commonly within the white matter.[51] More than three-quarters of cases are believed to result from issues that occur during pregnancy.[51] Most children who are born with cerebral palsy have more than one risk factor associated with CP.[52]
While in certain cases there is no identifiable cause, typical causes include problems in intrauterine development (e.g. exposure to radiation, infection, fetal growth restriction), hypoxia of the brain (thrombotic events, placental conditions), birth trauma during labor and delivery, and complications around birth or during childhood.[36][53]
In Africa birth asphyxia, high bilirubin levels, and infections in newborns of the central nervous system are main cause. Many cases of CP in Africa could be prevented with better resources available.[54]
Preterm birth
Between 40% and 50% of all children who develop cerebral palsy were born prematurely.[55] Most of these cases (75-90%) are believed due to issues that occur around the time of birth, often just after birth.[51] Multiple-birth infants are also more likely than single-birth infants to have CP.[56] They are also more likely to be born with a low birth weight.
In those who are born with a weight between 1 kg and 1.5 kg CP occurs in 6%.[2] Among those born before 28 weeks of gestation it occurs in 11%.[2] Genetic factors are believed to play an important role in prematurity and cerebral palsy generally.[57] While in those who are born between 34 and 37 weeks the risk is 0.4% (three times normal).[58]
Term infants
In babies that are born at term risk factors include problems with the placenta, birth defects, low birth weight, breathing meconium into the lungs, a delivery requiring either the use of instruments or an emergency Caesarean section, birth asphyxia, seizures just after birth, respiratory distress syndrome, low blood sugar, and infections in the baby.[59]
As of 2013, it was unclear how much of a role birth asphyxia plays as a cause.[60] It is unclear if the size of the placenta plays a role.[61] As of 2015 it is evident that in advanced countries, most cases of cerebral palsy in term or near-term neonates have explanations other than asphyxia.[53]
Genetics
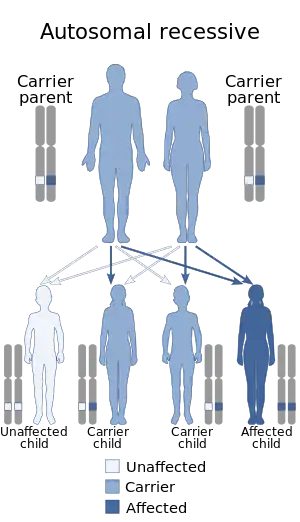
About 2% of all CP cases are inherited, with glutamate decarboxylase-1 being one of the possible enzymes involved.[5] Most inherited cases are autosomal recessive.[5]
Early childhood
After birth, other causes include toxins, severe jaundice,[62] lead poisoning, physical brain injury, stroke,[63] abusive head trauma, incidents involving hypoxia to the brain (such as near drowning), and encephalitis or meningitis.[62]
Others
Infections in the mother, even those not easily detected, can triple the risk of the child developing cerebral palsy .[64] Infections of the fetal membranes known as chorioamnionitis increases the risk.[65]
Intrauterine and neonatal insults (many of which are infectious) increase the risk.[66]
It has been hypothesised that some cases of cerebral palsy are caused by the death in very early pregnancy of an identical twin.[67]
Rh blood type incompatibility can cause the mother's immune system to attack the baby's red blood cells.[1]
Diagnosis
The diagnosis of cerebral palsy has historically rested on the person's history and physical examination. A general movements assessment, which involves measuring movements that occur spontaneously among those less than four months of age, appears most accurate.[68][69] Children who are more severely affected are more likely to be noticed and diagnosed earlier. Abnormal muscle tone, delayed motor development and persistence of primitive reflexes are the main early symptoms of CP.[30] Symptoms and diagnosis typically occur by the age of 2,[70] although persons with milder forms of cerebral palsy may be over the age of 5, if not in adulthood, when finally diagnosed.[71] Early diagnosis and intervention are seen as being a key part of managing cerebral palsy.[72] It is a developmental disability.[68]
Once a person is diagnosed with cerebral palsy, further diagnostic tests are optional. Neuroimaging with CT or MRI is warranted when the cause of a person's cerebral palsy has not been established. An MRI is preferred over CT, due to diagnostic yield and safety. When abnormal, the neuroimaging study can suggest the timing of the initial damage. The CT or MRI is also capable of revealing treatable conditions, such as hydrocephalus, porencephaly, arteriovenous malformation, subdural hematomas and hygromas, and a vermian tumour[73] (which a few studies suggest are present 5–22% of the time). Furthermore, an abnormal neuroimaging study indicates a high likelihood of associated conditions, such as epilepsy and intellectual disability.[74] There is a small risk associated with sedating children in facilitate a clear MRI.[71]
The age when CP is diagnosed is important, but medical professionals disagree over the best age to make the diagnosis.[69] The earlier CP is diagnosed correctly, the better the opportunities are to provide the child with physical and educational help, but there might be a greater chance of confusing CP with another problem, especially if the child is 18 months of age or younger.[69] Infants may have temporary problems with muscle tone or control that can be confused with CP, which is permanent.[69] A metabolism disorder or tumors in the nervous system may appear to be CP; metabolic disorders, in particular, can produce brain problems that look like CP on an MRI.[1] Disorders that deteriorate the white matter in the brain and problems that cause spasms and weakness in the legs, may be mistaken for CP if they first appear early in life.[69] However, these disorders get worse over time, and CP does not[69] (although it may change in character).[1] In infancy it may not be possible to tell the difference between them.[69] In the UK, not being able to sit independently by the age of 8 months is regarded as a clinical sign for further monitoring.[71] Fragile X syndrome (a cause of autism and intellectual disability) and general intellectual disability must also be ruled out.[69] Cerebral palsy specialist John McLaughlin recommends waiting until the child is 36 months of age before making a diagnosis, because by that age, motor capacity is easier to assess.[69]
Classification
CP is classified by the types of motor impairment of the limbs or organs, and by restrictions to the activities an affected person may perform.[75] The Gross Motor Function Classification System-Expanded and Revised and the Manual Ability Classification System are used to describe mobility and manual dexterity in people with cerebral palsy, and recently the Communication Function Classification System, and the Eating and Drinking Ability Classification System have been proposed to describe those functions.[76] There are three main CP classifications by motor impairment: spastic, ataxic, and dyskinetic. Additionally, there is a mixed type that shows a combination of features of the other types. These classifications reflect the areas of the brain that are damaged.
Cerebral palsy is also classified according to the topographic distribution of muscle spasticity.[77] This method classifies children as diplegic, (bilateral involvement with leg involvement greater than arm involvement), hemiplegic (unilateral involvement), or quadriplegic (bilateral involvement with arm involvement equal to or greater than leg involvement).[78][77]
Spastic
Spastic cerebral palsy is the type of cerebral palsy characterized by spasticity or high muscle tone often resulting in stiff, jerky movements.[79] Itself an umbrella term encompassing spastic hemiplegia, spastic diplegia, spastic quadriplegia and — where solely one limb or one specific area of the body is affected— spastic monoplegia. Spastic cerebral palsy affects the motor cortex[79] of the brain, a specific portion of the cerebral cortex responsible for the planning and completion of voluntary movement.[80] Spastic CP is the most common type of overall cerebral palsy, representing about 80% of cases.[81] Botulinum toxin is effective in decreasing spasticity. It can help increase range of motion which could help mitigate CPs effects on the growing bones of children. There is an improvement in motor functions in the children and ability to walk.[82]
Ataxic
Ataxic cerebral palsy is observed in approximately 5-10% of all cases of cerebral palsy, making it the least frequent form of cerebral palsy.[83] Ataxic cerebral palsy is caused by damage to cerebellar structures.[84] Because of the damage to the cerebellum, which is essential for coordinating muscle movements and balance, patients with ataxic cerebral palsy experience problems in coordination, specifically in their arms, legs, and trunk. Ataxic cerebral palsy is known to decrease muscle tone.[85] The most common manifestation of ataxic cerebral palsy is intention (action) tremor, which is especially apparent when carrying out precise movements, such as tying shoe laces or writing with a pencil. This symptom gets progressively worse as the movement persists, making the hand shake. As the hand gets closer to accomplishing the intended task, the trembling intensifies, which makes it even more difficult to complete.[78]
Dyskinetic
Dyskinetic cerebral palsy (sometimes abbreviated DCP) is primarily associated with damage to the basal ganglia and the substantia nigra in the form of lesions that occur during brain development due to bilirubin encephalopathy and hypoxic-ischemic brain injury.[86] DCP is characterized by both hypertonia and hypotonia, due to the affected individual's inability to control muscle tone.[78] Clinical diagnosis of DCP typically occurs within 18 months of birth and is primarily based upon motor function and neuroimaging techniques.[87][88] Dyskinetic cerebral palsy is a extrapyramidal form of cerebral palsy.[89] Dyskinetic cerebral palsy can be divided into two different groups; choreoathetosis and dystonia.[78] Choreo-athetotic CP is characterized by involuntary movements, whereas dystonic CP is characterized by slow, strong contractions, which may occur locally or encompass the whole body.[77]
Mixed
Mixed cerebral palsy has symptoms of dyskinetic, ataxic and spastic CP appearing simultaneously, each to varying degrees, and both with and without symptoms of each. Mixed CP is the most difficult to treat as it is extremely heterogeneous and sometimes unpredictable in its symptoms and development over the lifespan.
Prevention
Because the causes of CP are varied, a broad range of preventive interventions have been investigated.[90]
Electronic fetal monitoring has not helped to prevent CP, and in 2014 the American College of Obstetricians and Gynecologists, the Royal Australian and New Zealand College of Obstetricians and Gynaecologists, and the Society of Obstetricians and Gynaecologists of Canada have acknowledged that there are no long-term benefits of electronic fetal monitoring.[53] Prior to this, electronic fetal monitoring was widely used to prop up obstetric litigation.[91]
In those at risk of an early delivery, magnesium sulphate appears to decrease the risk of cerebral palsy.[92] It is unclear if it helps those who are born at term.[93] In those at high risk of preterm labor a review found that moderate to severe CP was reduced by the administration of magnesium sulphate, and that adverse effects on the babies from the magnesium sulphate were not significant. Mothers who received magnesium sulphate could experience side effects such as respiratory depression and nausea.[94] However, guidelines for the use of magnesium sulfate in mothers at risk of preterm labour are not strongly adhered to.[95] Caffeine is used to treat apnea of prematurity and reduces the risk of cerebral palsy in premature babies, but there are also concerns of long term negative effects.[96] A moderate quality level of evidence indicates that giving women antibiotics during preterm labor before her membranes have ruptured (water is not yet not broken) may increase the risk of cerebral palsy for the child.[97] Additionally, for preterm babies for whom there is a chance of fetal compromise, allowing the birth to proceed rather than trying to delay the birth may lead to an increased risk of cerebral palsy in the child.[97] Corticosteroids are sometimes taken by pregnant women expecting a preterm birth to provide neuroprotection to their baby.[98] Taking corticosteroids during pregnancy is shown to have no significant correlation with developing cerebral palsy in preterm births.[97]
Cooling high-risk full-term babies shortly after birth may reduce disability,[99] but this may only be useful for some forms of the brain damage that causes CP.[70]
Management

Over time, the approach to CP management has shifted away from narrow attempts to fix individual physical problems – such as spasticity in a particular limb – to making such treatments part of a larger goal of maximizing the person's independence and community engagement.[100]: 886 However, the evidence base for the effectiveness of intervention programs reflecting the philosophy of independence has not yet caught up: effective interventions for body structures and functions have a strong evidence base, but evidence is lacking for effective interventions targeted toward participation, environment, or personal factors.[100] There is also no good evidence to show that an intervention that is effective at the body-specific level will result in an improvement at the activity level, or vice versa.[100] Although such cross-over benefit might happen, not enough high-quality studies have been done to demonstrate it.[100]
Because cerebral palsy has "varying severity and complexity" across the lifespan,[76] it can be considered a collection of conditions for management purposes.[70] A multidisciplinary approach for cerebral palsy management is recommended,[76] focusing on "maximising individual function, choice and independence" in line with the International Classification of Functioning, Disability and Health's goals.[71] The team may include a paediatrician, a health visitor, a social worker, a physiotherapist, an orthotist, a speech and language therapist, an occupational therapist, a teacher specialising in helping children with visual impairment, an educational psychologist, an orthopaedic surgeon, a neurologist and a neurosurgeon.[101]
Various forms of therapy are available to people living with cerebral palsy as well as caregivers and parents. Treatment may include one or more of the following: physical therapy; occupational therapy; speech therapy; water therapy; drugs to control seizures, alleviate pain, or relax muscle spasms (e.g. benzodiazepines); surgery to correct anatomical abnormalities or release tight muscles; braces and other orthotic devices; rolling walkers; and communication aids such as computers with attached voice synthesisers. A Cochrane review published in 2004 found a trend toward benefit of speech and language therapy for children with cerebral palsy, but noted the need for high quality research.[102] A 2013 systematic review found that many of the therapies used to treat CP have no good evidence base; the treatments with the best evidence are medications (anticonvulsants, botulinum toxin, bisphosphonates, diazepam), therapy (bimanual training, casting, constraint-induced movement therapy, context-focused therapy, fitness training, goal-directed training, hip surveillance, home programmes, occupational therapy after botulinum toxin, pressure care) and surgery. Surgical intervention in CP children mainly includes orthopaedic surgery and neurosurgery (selective dorsal rhizotomy).[100]
Prognosis
CP is not a progressive disorder (meaning the brain damage does not worsen), but the symptoms can become more severe over time. A person with the disorder may improve somewhat during childhood if he or she receives extensive care, but once bones and musculature become more established, orthopedic surgery may be required. People with CP can have varying degrees of cognitive impairment or none whatsoever. The full intellectual potential of a child born with CP is often not known until the child starts school. People with CP are more likely to have learning disorders, but have normal intelligence. Intellectual level among people with CP varies from genius to intellectually disabled, as it does in the general population, and experts have stated that it is important not to underestimate the capabilities of a person with CP and to give them every opportunity to learn.[103]
The ability to live independently with CP varies widely, depending partly on the severity of each person's impairment and partly on the capability of each person to self-manage the logistics of life. Some individuals with CP require personal assistant services for all activities of daily living. Others only need assistance with certain activities, and still others do not require any physical assistance. But regardless of the severity of a person's physical impairment, a person's ability to live independently often depends primarily on the person's capacity to manage the physical realities of his or her life autonomously. In some cases, people with CP recruit, hire, and manage a staff of personal care assistants (PCAs). PCAs facilitate the independence of their employers by assisting them with their daily personal needs in a way that allows them to maintain control over their lives.
Puberty in young adults with cerebral palsy may be precocious or delayed. Delayed puberty is thought to be a consequence of nutritional deficiencies.[104] There is currently no evidence that CP affects fertility, although some of the secondary symptoms have been shown to affect sexual desire and performance.[105] Adults with CP were less likely to get routine reproductive health screening as of 2005. Gynecological examinations may have to be performed under anesthesia due to spasticity, and equipment is often not accessible. Breast self-examination may be difficult, so partners or carers may have to perform it. Women with CP reported higher levels of spasticity and urinary incontinence during menstruation in a study. Men with CP have higher levels of cryptorchidism at the age of 21.[104]
CP can significantly reduce a person's life expectancy, depending on the severity of their condition and the quality of care they receive.[51][106] 5-10% of children with CP die in childhood, particularly where seizures and intellectual disability also affect the child.[76] The ability to ambulate, roll, and self-feed has been associated with increased life expectancy.[107] While there is a lot of variation in how CP affects people, it has been found that "independent gross motor functional ability is a very strong determinant of life expectancy".[108] According to the Australian Bureau of Statistics, in 2014, 104 Australians died of cerebral palsy.[109] The most common causes of death in CP are related to respiratory causes, but in middle age cardiovascular issues and neoplastic disorders become more prominent.[110]
Self-care
For many children with CP, parents are heavily involved in self-care activities. Self-care activities, such as bathing, dressing, grooming, can be difficult for children with CP as self-care depends primarily on use of the upper limbs.[111] For those living with CP, impaired upper limb function affects almost 50% of children and is considered the main factor contributing to decreased activity and participation.[112] As the hands are used for many self-care tasks, sensory and motor impairments of the hands make daily self-care more difficult.[31][113] Motor impairments cause more problems than sensory impairments.[31] The most common impairment is that of finger dexterity, which is the ability to manipulate small objects with the fingers.[31] Compared to other disabilities, people with cerebral palsy generally need more help in performing daily tasks.[114] Occupational therapists are healthcare professionals that help individuals with disabilities gain or regain their independence through the use of meaningful activities.[115]
Productivity
The effects of sensory, motor and cognitive impairments affect self-care occupations in children with CP and productivity occupations. Productivity can include, but is not limited to, school, work, household chores or contributing to the community.[116]
Play is included as a productive occupation as it is often the primary activity for children.[117] If play becomes difficult due to a disability, like CP, this can cause problems for the child.[118] These difficulties can affect a child's self-esteem.[118] In addition, the sensory and motor problems experienced by children with CP affect how the child interacts with their surroundings, including the environment and other people.[118] Not only do physical limitations affect a child's ability to play, the limitations perceived by the child's caregivers and playmates also affect the child's play activities.[119] Some children with disabilities spend more time playing by themselves.[120] When a disability prevents a child from playing, there may be social, emotional and psychological problems,[121] which can lead to increased dependence on others, less motivation, and poor social skills.[122]
In school, students are asked to complete many tasks and activities, many of which involve handwriting. Many children with CP have the capacity to learn and write in the school environment.[123] However, students with CP may find it difficult to keep up with the handwriting demands of school and their writing may be difficult to read.[123] In addition, writing may take longer and require greater effort on the student's part.[123] Factors linked to handwriting include postural stability, sensory and perceptual abilities of the hand, and writing tool pressure.[123]
Speech impairments may be seen in children with CP depending on the severity of brain damage.[124] Communication in a school setting is important because communicating with peers and teachers is very much a part of the "school experience" and enhances social interaction. Problems with language or motor dysfunction can lead to underestimating a student's intelligence.[125] In summary, children with CP may experience difficulties in school, such as difficulty with handwriting, carrying out school activities, communicating verbally and interacting socially.
Leisure
Leisure activities can have several positive effects on physical health, mental health, life satisfaction and psychological growth for people with physical disabilities like CP.[126] Common benefits identified are stress reduction, development of coping skills, companionship, enjoyment, relaxation and a positive effect on life satisfaction.[127] In addition, for children with CP, leisure appears to enhance adjustment to living with a disability.[127]
Leisure can be divided into structured (formal) and unstructured (informal) activities.[128] Children and teens with CP engage in less habitual physical activity than their peers.[129] Children with CP primarily engage in physical activity through therapies aimed at managing their CP, or through organized sport for people with disabilities.[130] It is difficult to sustain behavioural change in terms of increasing physical activity of children with CP.[131] Gender, manual dexterity, the child's preferences, cognitive impairment and epilepsy were found to affect children's leisure activities, with manual dexterity associated with more leisure activity.[132] Although leisure is important for children with CP, they may have difficulties carrying out leisure activities due to social and physical barriers.
Children with cerebral palsy may face challenges when it comes to participating in sports. This comes with being discouraged from physical activity because of these perceived limitations imposed by their medical condition.[133]
Participation and barriers
Participation is involvement in life situations and everyday activities.[134] Participation includes self-care, productivity, and leisure. In fact, communication, mobility, education, home life, leisure and social relationships require participation, and indicate the extent to which children function in their environment.[134] Barriers can exist on three levels: micro, meso and macro.[135] First, the barriers at the micro level involve the person.[135] Barriers at the micro level include the child's physical limitations (motor, sensory and cognitive impairments) or their subjective feelings regarding their ability to participate.[136] For example, the child may not participate in group activities due to lack of confidence. Second, barriers at the meso level include the family and community.[135] These may include negative attitudes of people toward disability or lack of support within the family or in the community.[137] One of the main reasons for this limited support appears to be the result of a lack of awareness and knowledge regarding the child's ability to engage in activities despite his or her disability.[137] Third, barriers at the macro level incorporate the systems and policies that are not in place or hinder children with CP. These may be environmental barriers to participation such as architectural barriers, lack of relevant assistive technology and transportation difficulties due to limited wheelchair access or public transit that can accommodate children with CP.[137] For example, a building without an elevator can prevent the child from accessing higher floors.
A 2013 review stated that outcomes for adults with cerebral palsy without intellectual disability in the 2000s were that "60–80% completed high school, 14–25% completed college, up to 61% were living independently in the community, 25–55% were competitively employed, and 14–28% were involved in long term relationships with partners or had established families".[138] Adults with cerebral palsy may not seek physical therapy due to transport issues, financial restrictions and practitioners not feeling like they know enough about cerebral palsy to take people with CP on as clients.[139]
A study in young adults (18–34) on transitioning to adulthood found that their concerns were physical health care and understanding their bodies, being able to navigate and use services and supports successfully, and dealing with prejudices. A feeling of being "thrust into adulthood" was common in the study.[140]
Aging
Children with CP may not successfully transition into using adult services because they are not referred to one upon turning 18, and may decrease their use of services.[110] Because children with cerebral palsy are often told that it is a non-progressive disease, they may be unprepared for the greater effects of the aging process as they head into their 30s.[141] Young adults with cerebral palsy experience problems with aging that able-bodied adults experience "much later in life".[23]: 42 25% or more adults with cerebral palsy who can walk experience increasing difficulties walking with age.[142] Hand function does not seem to suffer similar declines.[50] Chronic disease risk, such as obesity, is also higher among adults with cerebral palsy than the general population.[143] Common problems include increased pain, reduced flexibility, increased spasms and contractures, post-impairment syndrome,[144] and increasing problems with balance.[33] Increased fatigue is also a problem.[145] When adulthood and cerebral palsy is discussed, as of 2011, it is not discussed in terms of the different stages of adulthood.[145]
Like they did in childhood, adults with cerebral palsy experience psychosocial issues related to their CP, chiefly the need for social support, self-acceptance, and acceptance by others. Workplace accommodations may be needed to enhance continued employment for adults with CP as they age. Rehabilitation or social programs that include salutogenesis may improve the coping potential of adults with CP as they age.[146]
Epidemiology
Cerebral palsy occurs in about 2.1 per 1000 live births.[2] In those born at term rates are lower at 1 per 1000 live births.[51] Rates appear to be similar in both the developing and developed world.[51] Within a population it may occur more often in poorer people.[147] The rate is higher in males than in females; in Europe it is 1.3 times more common in males.[148]
There was a "moderate, but significant" rise in the prevalence of CP between the 1970s and 1990s. This is thought to be due to a rise in low birth weight of infants and the increased survival rate of these infants. The increased survival rate of infants with CP in the 1970s and 80s may be indirectly due to the disability rights movement challenging perspectives around the worth of infants with disability, as well as the Baby Doe Law.[149]
As of 2005, advances in care of pregnant mothers and their babies has not resulted in a noticeable decrease in CP. This is generally attributed to medical advances in areas related to the care of premature babies (which results in a greater survival rate). Only the introduction of quality medical care to locations with less-than-adequate medical care has shown any decreases. The incidence of CP increases with premature or very low-weight babies regardless of the quality of care.[150] As of 2016, there is a suggestion that both incidence and severity are slightly decreasing – more research is needed to find out if this is significant, and if so, which interventions are effective.[90]
Prevalence of cerebral palsy is best calculated around the school entry age of about 6 years, the prevalence in the U.S. is estimated to be 2.4 out of 1000 children.[151]
History
Cerebral palsy has affected humans since antiquity. A decorated grave marker dating from around the 15th to 14th century BCE shows a figure with one small leg and using a crutch, possibly due to cerebral palsy. The oldest likely physical evidence of the condition comes from the mummy of Siptah, an Egyptian Pharaoh who ruled from about 1196 to 1190 BCE and died at about 20 years of age. The presence of cerebral palsy has been suspected due to his deformed foot and hands.[7]
The medical literature of the ancient Greeks discusses paralysis and weakness of the arms and legs; the modern word palsy comes from the Ancient Greek words παράλυση or πάρεση, meaning paralysis or paresis respectively. The works of the school of Hippocrates (460–c. 370 BCE), and the manuscript On the Sacred Disease in particular, describe a group of problems that matches up very well with the modern understanding of cerebral palsy. The Roman Emperor Claudius (10 BCE–54 CE) is suspected of having CP, as historical records describe him as having several physical problems in line with the condition. Medical historians have begun to suspect and find depictions of CP in much later art. Several paintings from the 16th century and later show individuals with problems consistent with it, such as Jusepe de Ribera's 1642 painting The Clubfoot.[7]
The modern understanding of CP as resulting from problems within the brain began in the early decades of the 1800s with a number of publications on brain abnormalities by Johann Christian Reil, Claude François Lallemand and Philippe Pinel. Later physicians used this research to connect problems in the brain with specific symptoms. The English surgeon William John Little (1810–1894) was the first person to study CP extensively. In his doctoral thesis he stated that CP was a result of a problem around the time of birth. He later identified a difficult delivery, a preterm birth and perinatal asphyxia in particular as risk factors. The spastic diplegia form of CP came to be known as Little's disease.[7] At around this time, a German surgeon was also working on cerebral palsy, and distinguished it from polio.[152] In the 1880s British neurologist William Gowers built on Little's work by linking paralysis in newborns to difficult births. He named the problem "birth palsy" and classified birth palsies into two types: peripheral and cerebral.[7]
Working in Pennsylvania in the 1880s, Canadian-born physician William Osler (1849–1919) reviewed dozens of CP cases to further classify the disorders by the site of the problems on the body and by the underlying cause. Osler made further observations tying problems around the time of delivery with CP, and concluded that problems causing bleeding inside the brain were likely the root cause. Osler also suspected polioencephalitis as an infectious cause. Through the 1890s, scientists commonly confused CP with polio.[7]
Before moving to psychiatry, Austrian neurologist Sigmund Freud (1856–1939) made further refinements to the classification of the disorder. He produced the system still being used today. Freud's system divides the causes of the disorder into problems present at birth, problems that develop during birth, and problems after birth. Freud also made a rough correlation between the location of the problem inside the brain and the location of the affected limbs on the body, and documented the many kinds of movement disorders.[7]
In the early 20th century, the attention of the medical community generally turned away from CP until orthopedic surgeon Winthrop Phelps became the first physician to treat the disorder. He viewed CP from a musculoskeletal perspective instead of a neurological one. Phelps developed surgical techniques for operating on the muscles to address issues such as spasticity and muscle rigidity. Hungarian physical rehabilitation practitioner András Pető developed a system to teach children with CP how to walk and perform other basic movements. Pető's system became the foundation for conductive education, widely used for children with CP today. Through the remaining decades, physical therapy for CP has evolved, and has become a core component of the CP management program.[7]
In 1997, Robert Palisano et al. introduced the Gross Motor Function Classification System (GMFCS) as an improvement over the previous rough assessment of limitation as either mild, moderate or severe.[75] The GMFCS grades limitation based on observed proficiency in specific basic mobility skills such as sitting, standing and walking, and takes into account the level of dependency on aids such as wheelchairs or walkers. The GMFCS was further revised and expanded in 2007.[75]
Society and culture
Economic impact
It is difficult to directly compare the cost and cost-effectiveness of interventions to prevent cerebral palsy or the cost of interventions to manage CP.[95] Access Economics has released a report on the economic impact of cerebral palsy in Australia. The report found that, in 2007, the financial cost of cerebral palsy (CP) in Australia was $AUS 1.47 billion or 0.14% of GDP.[153] Of this:
- $AUS 1.03 billion (69.9%) was productivity lost due to lower employment, absenteeism and premature death of Australians with CP
- $AUS 141 million (9.6%) was the DWL from transfers including welfare payments and taxation forgone
- $AUS 131 million (9.0%) was other indirect costs such as direct program services, aides and home modifications and the bringing-forward of funeral costs
- $AUS 129 million (8.8%) was the value of the informal care for people with CP
- $AUS 40 million (2.8%) was direct health system expenditure
The value of lost well-being (disability and premature death) was a further $AUS 2.4 billion.
In per capita terms, this amounts to a financial cost of $AUS 43,431 per person with CP per annum. Including the value of lost well-being, the cost is over $115,000 per person per annum.
Individuals with CP bear 37% of the financial costs, and their families and friends bear a further 6%. Federal government bears around one-third (33%) of the financial costs (mainly through taxation revenues forgone and welfare payments). State governments bear under 1% of the costs, while employers bear 5% and the rest of society bears the remaining 19%. If the burden of disease (lost well-being) is included, individuals bear 76% of the costs.
The average lifetime cost for people with CP in the US is $US921,000 per individual, including lost income.[154]
In the United States many states allow Medicaid beneficiaries to use their Medicaid funds to hire their own PCAs, instead of forcing them to use institutional or managed care.[155]
In India, the government-sponsored program called "NIRAMAYA" for the medical care of children with neurological and muscular deformities has proved to be an ameliorating economic measure for persons with such disabilities.[156] It has shown that persons with mental or physically debilitating congenital disabilities can lead better lives if they have financial independence.[157]
Use of the term
"Cerebral" means "of, or pertaining to, the cerebrum or the brain"[158] and "palsy" means "paralysis, generally partial, whereby a local body area is incapable of voluntary movement".[159] It has been proposed to change the name to "cerebral palsy spectrum disorder" to reflect the diversity of presentations of CP.[160]
Many people would rather be referred to as a person with a disability (people-first language) instead of as handicapped. "Cerebral Palsy: A Guide for Care" at the University of Delaware offers the following guidelines:
Impairment is the correct term to use to define a deviation from normal, such as not being able to make a muscle move or not being able to control an unwanted movement. Disability is the term used to define a restriction in the ability to perform a normal activity of daily living which someone of the same age is able to perform. For example, a three-year-old child who is not able to walk has a disability because a normal three-year-old can walk independently. A handicapped child or adult is one who, because of the disability, is unable to achieve the normal role in society commensurate with his age and socio-cultural milieu. As an example, a sixteen-year-old who is unable to prepare his own meal or care for his own toilet or hygiene needs is handicapped. On the other hand, a sixteen-year-old who can walk only with the assistance of crutches but who attends a regular school and is fully independent in activities of daily living is disabled but not handicapped. All disabled people are impaired, and all handicapped people are disabled, but a person can be impaired and not necessarily be disabled, and a person can be disabled without being handicapped.[161]
The term "spastic" denotes the attribute of spasticity in types of spastic CP. In 1952 a UK charity called The Spastics Society was formed.[162] The term "spastics" was used by the charity as a term for people with CP. The word "spastic" has since been used extensively as a general insult to disabled people, which some see as extremely offensive. They are also frequently used to insult able-bodied people when they seem overly uncoordinated, anxious, or unskilled in sports. The charity changed its name to Scope in 1994.[162] In the United States the word spaz has the same usage as an insult, but is not generally associated with CP.[163]
Media
Maverick documentary filmmaker Kazuo Hara criticises the mores and customs of Japanese society in an unsentimental portrait of adults with cerebral palsy in his 1972 film Goodbye CP (Sayonara CP). Focusing on how people with cerebral palsy are generally ignored or disregarded in Japan, Hara challenges his society's taboos about physical handicaps. Using a deliberately harsh style, with grainy black-and-white photography and out-of-sync sound, Hara brings a stark realism to his subject.[164]
Spandan (2012), a film by Vegitha Reddy and Aman Tripathi, delves into the dilemma of parents whose child has cerebral palsy. While films made with children with special needs as central characters have been attempted before, the predicament of parents dealing with the stigma associated with the condition and beyond is dealt in Spandan. In one of the songs of Spandan "Chal chaal chaal tu bala" more than 50 CP kids have acted. The famous classical singer Devaki Pandit has given her voice to the song penned by Prof. Jayant Dhupkar and composed by National Film Awards winner Isaac Thomas Kottukapally.[165][166][167][168]
My Left Foot (1989) is a drama film directed by Jim Sheridan and starring Daniel Day-Lewis. It tells the true story of Christy Brown, an Irishman born with cerebral palsy, who could control only his left foot. Christy Brown grew up in a poor, working-class family, and became a writer and artist. It won the Academy Award for Best Actor (Daniel Day-Lewis) and Best Actress in a Supporting Role (Brenda Fricker). It was also nominated for Best Director, Best Picture and Best Writing, Screenplay Based on Material from Another Medium. It also won the New York Film Critics Circle Award for Best Film for 1989.[169]
Call the Midwife (2012–) has featured two episodes with actor Colin Young, who he himself has cerebral palsy, playing a character with the same disability. His story lines have focused on the segregation of those with disabilities in the UK in the 1950s, and also romantic relationships between people with disabilities.[170]
Micah Fowler, an American actor with CP, stars in the ABC sitcom Speechless (2016–19), which explores both the serious and humorous challenges a family faces with a teenager with CP.[171]
Special (2019) is a comedy series that premiered on Netflix on 12 April 2019. It was written, produced and stars Ryan O'Connell as a young gay man with mild cerebral palsy. It is based on O'Connell's book I'm Special: And Other Lies We Tell Ourselves.[172]
Notable cases
- Harold Elwood Yuker, a psychologist and educator at Hofstra University[173]
- Two sons of Canadian rock musician Neil Young, Zeke and Ben[174] In 1986, Young helped found the Bridge School, an educational organization for children with severe verbal and physical disabilities, and its annual supporting Bridge School Benefit concerts, together with his wife Pegi.[175][176]
- Geri Jewell had a regular role in the prime-time series The Facts of Life.[177]
- Josh Blue, winner of the fourth season of NBC's Last Comic Standing, whose act revolves around his CP.[178] Blue was also on the 2004 U.S. Paralympic soccer team.[179]
- Jason Benetti, Play-by-play broadcaster for ESPN, Fox Sports, Westwood One, and Time Warner covering football, baseball, lacrosse, hockey, and basketball. Since 2016, he is also the television play-by-play announcer for Chicago White Sox home games.[180]
- Jack Carroll, British comedian and runner-up in the seventh season of Britain's Got Talent.[181]
- Abbey Curran, American beauty queen who represented Iowa at Miss USA 2008 and was the first contestant with a disability to compete.[182]
- Francesca Martinez, British stand-up comedian and actress.[183]
- Evan O'Hanlon, Australian Paralympian, the fastest athlete with cerebral palsy in the world.[184]
- Arun Shourie's son Aditya about whom he has written a book Does He Know a Mother's Heart[185]
- Maysoon Zayid, the self-described "Palestinian Muslim virgin with cerebral palsy, from New Jersey", who is an actress, stand-up comedian and activist.[186] Zayid has been a resident of Cliffside Park, New Jersey.[187] She is considered one of America's first Muslim women comedians and the first person ever to perform standup in Palestine and Jordan.[188]
- RJ Mitte, an American actor best known for his role as Walter White Jr. in Breaking Bad. He is also a celebrity ambassador for United Cerebral Palsy.[189]
- Zach Anner, an American comedian, actor and writer. He had a television series on Oprah Winfrey's OWN called Rollin' With Zach and is the author of If at Birth You Don't Succeed.[190]
- Kaine, a member of the popular Atlanta, Georgia-based hip-hop duo The Ying Yang Twins, has a mild form of cerebral palsy that causes him to limp.[191]
- Hannah Cockroft is a British wheelchair athlete specialising in sprint distances in the T34 classification. She holds the Paralympic and world records for the 100 metres, 200 metres and 400 metres in her classification.[192][193][194]
- Keah Brown, disability rights activist, author, journalist, and writer.[195]
Litigation
Because of the false perception that cerebral palsy is mostly caused by trauma during birth, as of 2005, 60% of obstetric litigation was about cerebral palsy, which Alastair MacLennan, Professor of Obstetrics and Gynaecology at the University of Adelaide, regards as causing an exodus from the profession.[196] In the latter half of the 20th century, obstetric litigation about the cause of cerebral palsy became more common, leading to defensive medicine being practiced.[91]
See also
- Cerebral palsy sport classification – describes the disability sport classification for cerebral palsy.
- Inclusive recreation
- World Cerebral Palsy Day
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 "Cerebral Palsy: Hope Through Research". National Institute of Neurological Disorders and Stroke. July 2013. Archived from the original on 21 February 2017. Retrieved 21 February 2017.
- 1 2 3 4 5 Oskoui, M; Coutinho, F; Dykeman, J; Jetté, N; Pringsheim, T (June 2013). "An update on the prevalence of cerebral palsy: a systematic review and meta-analysis". Developmental Medicine & Child Neurology. 55 (6): 509–19. doi:10.1111/dmcn.12080. PMID 23346889.
- ↑ Haak, Peterson; Lenski, Madeleine; Hidecker, Mary Jo Cooley; Li, Min; Paneth, Nigel (October 2009). "Cerebral palsy and aging". Developmental Medicine & Child Neurology. 51: 16–23. doi:10.1111/j.1469-8749.2009.03428.x. PMC 4183123. PMID 19740206.
- ↑ "Cerebral Palsy: Overview". National Institutes of Health. Archived from the original on 15 February 2017. Retrieved 21 February 2017.
- 1 2 3 "CEREBRAL PALSY, SPASTIC QUADRIPLEGIC, 1; CPSQ1". Online Mendelian Inheritance in Man. 28 June 2016. Archived from the original on 1 October 2019. Retrieved 26 January 2018.
- ↑ "How many people are affected?". National Institutes of Health. 5 September 2014. Archived from the original on 2 April 2015. Retrieved 4 March 2015.
- 1 2 3 4 5 6 7 8 9 Panteliadis, C; Panteliadis, P; Vassilyadi, F (April 2013). "Hallmarks in the history of cerebral palsy: from antiquity to mid-20th century". Brain & Development. 35 (4): 285–92. doi:10.1016/j.braindev.2012.05.003. PMID 22658818.
- ↑ "What is cerebral palsy?". The Cerebral Palsied Association of the Philippines Inc. Archived from the original on 20 December 2016. Retrieved 4 December 2016.
- 1 2 Rosenbaum, P; Paneth, N; Leviton, A; Goldstein, M; Bax, M; Damiano, D; Dan, B; Jacobsson, B (2007). "A report: The definition and classification of cerebral palsy April 2006". Developmental Medicine & Child Neurology. 49 (s109): 8–14. doi:10.1111/j.1469-8749.2007.tb12610.x. PMID 17370477.; Corrected in Rosenbaum, P; Paneth, N; Leviton, A; Goldstein, M; Bax, M; Damiano, D; Dan, B; Jacobsson, B (2007). "A report: The definition and classification of cerebral palsy April 2006". Developmental Medicine & Child Neurology. 49 (6): 480. doi:10.1111/j.1469-8749.2007.00480.x.
- ↑ Kent R (2013). "Chapter 38: Cerebral Palsy". In Barnes MP, Good DC (eds.). Handbook of Clinical Neurology. 3. Vol. 110. Elsevier. pp. 443–459. ISBN 978-0444529015.
- ↑ Mathewson, Margie A.; Lieber, Richard L. (February 2015). "Pathophysiology of Muscle Contractures in Cerebral Palsy". Physical Medicine and Rehabilitation Clinics of North America. 26 (1): 57–67. doi:10.1016/j.pmr.2014.09.005. PMC 4258234. PMID 25479779.
- 1 2 3 4 5 El-Sobky, TA; Fayyad, TA; Kotb, AM; Kaldas, B (25 September 2017). "Bony reconstruction of hip in cerebral palsy children Gross Motor Function Classification System levels III to V: a systematic review". Journal of Pediatric Orthopedics. Part B. 27 (3): 221–230. doi:10.1097/BPB.0000000000000503. PMID 28953164.
- 1 2 Agarwal, Anil; Verma, Indreshwar (December 2012). "Cerebral palsy in children: An overview". Journal of Clinical Orthopaedics and Trauma. 3 (2): 77–81. doi:10.1016/j.jcot.2012.09.001. PMC 3872805. PMID 26403442.
- ↑ Smith, Martin; Kurian, Manju A. (September 2016). "The medical management of cerebral palsy". Paediatrics and Child Health (Submitted manuscript). 26 (9): 378–382. doi:10.1016/j.paed.2016.04.013. Archived from the original on 28 August 2021. Retrieved 1 August 2020.
- ↑ "Symptoms of Cerebral palsy". NHS Choices. NHS Gov.UK. 15 March 2017. Archived from the original on 7 April 2017. Retrieved 6 April 2017.
- ↑ Walshe, M; Smith, M; Pennington, L (14 November 2012). Walshe, Margaret (ed.). "Interventions for drooling in children with cerebral palsy". The Cochrane Database of Systematic Reviews. 11: CD008624. doi:10.1002/14651858.CD008624.pub3. PMID 23152263.
- 1 2 3 4 5 Sewell, M. D.; Eastwood, D. M.; Wimalasundera, N. (25 September 2014). "Managing common symptoms of cerebral palsy in children". The BMJ. 349 (sep25 7): g5474. doi:10.1136/bmj.g5474. PMID 25255910.
- ↑ Samijn, Bieke; Van Laecke, Erik; Renson, Catherine; Hoebeke, Piet; Plasschaert, Frank; Vande Walle, Johan; Van den Broeck, Christine (February 2016). "Lower urinary tract symptoms and urodynamic findings in children and adults with cerebral palsy: A systematic review". Neurourology and Urodynamics (Submitted manuscript). 36 (3): 541–549. doi:10.1002/nau.22982. PMID 26894322. Archived from the original on 24 September 2020. Retrieved 1 August 2020.
- ↑ Hinchcliffe, Archie; Rogers, Clare (2007). "Sensory integration problems in children with cerebral palsy". Children with Cerebral Palsy: a manual for therapists, parents and community workers (2nd ed., rev. ed.). New Delhi: SAGE Publications. ISBN 9788178299655.
- ↑ Alliance (UK), National Guideline (2019). Rationale and impact. National Institute for Health and Care Excellence (UK). Archived from the original on 9 March 2021. Retrieved 1 August 2020.
- 1 2 3 Mughal, M. Zulf (26 June 2014). "Fractures in Children with Cerebral Palsy". Current Osteoporosis Reports. 12 (3): 313–318. doi:10.1007/s11914-014-0224-1. PMID 24964775.
- ↑ Ozel, Sezgi; Switzer, Lauren; Macintosh, Alex; Fehlings, Darcy (September 2016). "Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: an update". Developmental Medicine & Child Neurology. 58 (9): 918–923. doi:10.1111/dmcn.13196. PMID 27435427.
- 1 2 Kerkovich, D, & Aisen, M 2009, 'Chapter 4: Cerebral Palsy', Medical Management of Adults with Neurologic Disabilities pp. 41–53 n.p.: Demos Medical Publishing, LLC. ISBN 9781933864457
- ↑ Veilleux, Louis-Nicolas; Rauch, Frank (30 August 2017). "Muscle-Bone Interactions in Pediatric Bone Diseases". Current Osteoporosis Reports. 15 (5): 425–432. doi:10.1007/s11914-017-0396-6. PMID 28856575.
- 1 2 Shore, Benjamin J.; White, Nathan; Kerr Graham, H. (August 2010). "Surgical correction of equinus deformity in children with cerebral palsy: a systematic review". Journal of Children's Orthopaedics. 4 (4): 277–290. doi:10.1007/s11832-010-0268-4. PMC 2908346. PMID 21804889.
- 1 2 Cloake, Thomas; Gardner, Adrian (December 2016). "The management of scoliosis in children with cerebral palsy: a review". Journal of Spine Surgery. 2 (4): 299–309. doi:10.21037/jss.2016.09.05. PMC 5233861. PMID 28097247.
- ↑ Rutz, Erich; Brunner, Reinald (November 2013). "Management of spinal deformity in cerebral palsy: conservative treatment". Journal of Children's Orthopaedics. 7 (5): 415–418. doi:10.1007/s11832-013-0516-5. PMC 3838520. PMID 24432104.
- ↑ Roberts, Andrew (September 2012). "The surgical treatment of cerebral palsy". Paediatrics and Child Health. 22 (9): 377–383. doi:10.1016/j.paed.2012.03.004.
- 1 2 Klingels, K.; De Cock, P.; Molenaers, G.; Desloovere, K.; Huenaerts, C.; Jaspers, E.; Feys, H. (2010). "Upper limb motor and sensory impairments in children with hemiplegic cerebral palsy. Can they be measured reliably?". Disability & Rehabilitation. 32 (5): 409–416. doi:10.3109/09638280903171469. PMID 20095955.
- 1 2 3 4 Robin C. Meyers; Steven J. Bachrach; Virginia A. Stallings (2017). "Cerebral Palsy". In Shirley W. Ekvall; Valli K. Ekvall (eds.). Pediatric and Adult Nutrition in Chronic Diseases, Developmental Disabilities, and Hereditary Metabolic Disorders: Prevention, Assessment, and Treatment. Oxford Scholarship Online. doi:10.1093/acprof:oso/9780199398911.003.0009. ISBN 9780199398911.
{{cite book}}: CS1 maint: url-status (link) - 1 2 3 4 5 Donkervoort, M.; Roebroeck, M.; Wiegerink, D.; Van der Heijden-Maessen, H.; Stam, H.; The Transition Research Group South (2007). "Determinants of functioning of adolescents and young adults with cerebral palsy". Disability & Rehabilitation. 29 (6): 453–463. doi:10.1080/09638280600836018. PMID 17364800.
- ↑ Bell, K L; Samson-Fang, L (December 2013). "Nutritional management of children with cerebral palsy". European Journal of Clinical Nutrition. 67: S13–S16. doi:10.1038/ejcn.2013.225. PMID 24301003.
- 1 2 Hirsh Adam T.; Gallegos Juan C.; Gertz Kevin J.; Engel Joyce M.; Jensen Mark P. (2010). "Symptom Burden in Individuals with Cerebral Palsy". Journal of Rehabilitation Research & Development. 47 (9): 863–67. doi:10.1682/jrrd.2010.03.0024. PMC 3158669. PMID 21174251.
- 1 2 Myrden, Andrew; Schudlo, Larissa; Weyand, Sabine; Zeyl, Timothy; Chau, Tom (August 2014). "Trends in Communicative Access Solutions for Children With Cerebral Palsy". Journal of Child Neurology. 29 (8): 1108–1118. doi:10.1177/0883073814534320. PMID 24820337.
- ↑ Love, Russell J.; Webb, Wanda G. (2013). Neurology for the Speech-Language Pathologist (2 ed.). Butterworth-Heinemann. p. 250. ISBN 9781483141992. Archived from the original on 9 December 2019. Retrieved 1 August 2020.
- 1 2 3 4 Beukelman, David R.; Mirenda, Pat (1999). Augmentative and Alternative Communication: Management of severe communication disorders in children and adults (2nd ed.). Baltimore: Paul H Brookes Publishing Co. pp. 246–249. doi:10.1080/07434619912331278735. ISBN 978-1-55766-333-7.
- ↑ McKearnan K.A.; Kieckhefer G.M.; Engel J.M.; Jensen M.P.; Labyak S. (2004). "Pain in children with cerebral palsy: A review". Journal of Neuroscience Nursing. 26 (5): 252–259. doi:10.1097/01376517-200410000-00004. PMID 15524243.
- ↑ Hauer, Julie; Houtrow, Amy J. (22 May 2017). "Pain Assessment and Treatment in Children With Significant Impairment of the Central Nervous System". Pediatrics. 139 (6): e20171002. doi:10.1542/peds.2017-1002. PMID 28562301.
- ↑ Blackman, James A; Svensson, Camilla I; Marchand, Serge (September 2018). "Pathophysiology of chronic pain in cerebral palsy: implications for pharmacological treatment and research". Developmental Medicine & Child Neurology. 60 (9): 861–865. doi:10.1111/dmcn.13930. PMID 29882358.
- ↑ Newman C.J.; O'Regan M.; Hensey O. (2006). "Sleep disorders in children with cerebral palsy". Developmental Medicine & Child Neurology. 48 (7): 564–8. doi:10.1017/S0012162206001198. PMID 16780625.
- ↑ Dutt, Risha; Roduta-Roberts, Mary; Brown, Cary (27 February 2015). "Sleep and Children with Cerebral Palsy: A Review of Current Evidence and Environmental Non-Pharmacological Interventions". Children. 2 (1): 78–88. doi:10.3390/children2010078. PMC 4928749. PMID 27417351.
- ↑ Stanton, Marion (2012). "Special Considerations". Understanding cerebral palsy : a guide for parents and professionals. London: Jessica Kingsley Publishers. p. 70. ISBN 9781849050609.
- ↑ Kingsnorth, S.; Orava, T.; Provvidenza, C.; Adler, E.; Ami, N.; Gresley-Jones, T.; Mankad, D.; Slonim, N.; Fay, L.; Joachimides, N.; Hoffman, A.; Hung, R.; Fehlings, D. (28 September 2015). "Chronic Pain Assessment Tools for Cerebral Palsy: A Systematic Review". Pediatrics. 136 (4): e947–e960. doi:10.1542/peds.2015-0273. PMID 26416940.
- ↑ Novak, I.; Hines, M.; Goldsmith, S.; Barclay, R. (8 October 2012). "Clinical Prognostic Messages From a Systematic Review on Cerebral Palsy". Pediatrics. 130 (5): e1285–e1312. doi:10.1542/peds.2012-0924. PMID 23045562.
- ↑ Jones, KB; Wilson, B; Weedon, D; Bilder, D (December 2015). "Care of Adults With Intellectual and Developmental Disabilities: Cerebral Palsy". FP Essentials. 439: 26–30. PMID 26669212.
- ↑ Krigger, KW (1 January 2006). "Cerebral palsy: an overview". American Family Physician. 73 (1): 91–100. PMID 16417071.
- ↑ Cremer, Nicole; Hurvitz, Edward A.; Peterson, Mark D. (January 2017). "Multimorbidity in Middle-Aged Adults with Cerebral Palsy". The American Journal of Medicine. 130 (6): 744.e9–744.e15. doi:10.1016/j.amjmed.2016.11.044. PMC 5502778. PMID 28065772.
- ↑ "Women's Health Initiative – Cerebral Palsy Foundation". Cerebral Palsy Foundation. 10 July 2015. Archived from the original on 24 December 2016. Retrieved 23 December 2016.
- ↑ Wimalasundera, Neil; Stevenson, Valerie L (June 2016). "Cerebral palsy". Practical Neurology. 16 (3): 184–194. doi:10.1136/practneurol-2015-001184. PMID 26837375.
- 1 2 van Gorp, Marloes; Hilberink, Sander R.; Noten, Suzie; Benner, Joyce L.; Stam, Henk J.; van der Slot, Wilma MA.; Roebroeck, Marij E. (February 2020). "The epidemiology of cerebral palsy in adulthood: A systematic review and meta-analysis of the most frequently studied outcomes". Archives of Physical Medicine and Rehabilitation. doi:10.1016/j.apmr.2020.01.009. PMID 32059945.
- 1 2 3 4 5 6 7 8 John Yarnell (2013). Epidemiology and Disease Prevention: A Global Approach (02 ed.). Oxford University Press. p. 190. ISBN 9780199660537. Archived from the original on 11 January 2021. Retrieved 1 August 2020.
- ↑ Eunson, Paul (September 2016). "Aetiology and epidemiology of cerebral palsy". Paediatrics and Child Health. 26 (9): 367–372. doi:10.1016/j.paed.2016.04.011.
- 1 2 3 Nelson KB, Blair E. (3 September 2015). "Prenatal Factors in Singletons with Cerebral Palsy Born at or near Term". The New England Journal of Medicine. 373 (10): 946–53. doi:10.1056/NEJMra1505261. PMID 26332549.
- ↑ Burton, Adrian (September 2015). "Fighting cerebral palsy in Africa". The Lancet Neurology. 14 (9): 876–877. doi:10.1016/S1474-4422(15)00189-1. PMID 26293560.
- ↑ William B. Carey, ed. (2009). Developmental-behavioral pediatrics (4th ed.). Philadelphia, PA: Saunders/Elsevier. p. 264. ISBN 9781416033707. Archived from the original on 7 March 2021. Retrieved 1 August 2020.
- ↑ Saunders, NR; Hellmann, J; Farine, D (October 2011). "Cerebral palsy and assisted conception". Journal of Obstetrics and Gynaecology Canada. 33 (10): 1038–43. doi:10.1016/s1701-2163(16)35053-8. PMID 22014781.
- ↑ Hallman, M (April 2012). "Premature birth and diseases in premature infants: common genetic background?". The Journal of Maternal-Fetal & Neonatal Medicine. 25 Suppl 1: 21–4. doi:10.3109/14767058.2012.667600. PMID 22385349.
- ↑ Poets, CF; Wallwiener, D; Vetter, K (October 2012). "Risks associated with delivering infants 2 to 6 weeks before term--a review of recent data". Deutsches Arzteblatt International. 109 (43): 721–6. doi:10.3238/arztebl.2012.0721. PMC 3498472. PMID 23181136.
- ↑ McIntyre, S; Taitz, D; Keogh, J; Goldsmith, S; Badawi, N; Blair, E (June 2013). "A systematic review of risk factors for cerebral palsy in children born at term in developed countries". Developmental Medicine & Child Neurology. 55 (6): 499–508. doi:10.1111/dmcn.12017. PMID 23181910.
- ↑ Ellenberg, JH; Nelson, KB (March 2013). "The association of cerebral palsy with birth asphyxia: a definitional quagmire". Developmental Medicine & Child Neurology. 55 (3): 210–6. doi:10.1111/dmcn.12016. PMID 23121164.
- ↑ Teng, J; Chang, T; Reyes, C; Nelson, KB (October 2012). "Placental weight and neurologic outcome in the infant: a review". The Journal of Maternal-Fetal & Neonatal Medicine. 25 (10): 2082–7. doi:10.3109/14767058.2012.671871. PMID 22394270.
- 1 2 "Cerebral Palsy | NCBDDD | CDC". Cerebral Palsy Home | NCBDDD | CDC. Archived from the original on 21 April 2017. Retrieved 20 April 2017.
- ↑ Kieffer, Sara. "Cerebral Palsy | Johns Hopkins Pediatric Neurosurgery". Archived from the original on 30 September 2015. Retrieved 18 September 2015.
- ↑ "Infection in the Newborn as a Cause of Cerebral Palsy, 12/2004". United Cerebral Palsy Research and Education Foundation (U.S.). Archived from the original on 29 July 2007. Retrieved 5 July 2007.
- ↑ Bersani, I; Thomas, W; Speer, CP (April 2012). "Chorioamnionitis—the good or the evil for neonatal outcome?". The Journal of Maternal-Fetal & Neonatal Medicine. 25 Suppl 1: 12–6. doi:10.3109/14767058.2012.663161. PMID 22309119.
- ↑ Mwaniki, MK; Atieno, M; Lawn, JE; Newton, CR (4 February 2012). "Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review". The Lancet. 379 (9814): 445–52. doi:10.1016/s0140-6736(11)61577-8. PMC 3273721. PMID 22244654.
- ↑ Pharoah PO (December 2005). "Causal hypothesis for some congenital anomalies". Twin Research and Human Genetics. 8 (6): 543–550. doi:10.1375/183242705774860141. PMID 16354495.
- 1 2 McIntyre, S; Morgan, C; Walker, K; Novak, I (November 2011). "Cerebral palsy—don't delay". Developmental Disabilities Research Reviews. 17 (2): 114–29. doi:10.1002/ddrr.1106. PMID 23362031.
- 1 2 3 4 5 6 7 8 9 Bosanquet, M; Copeland, L; Ware, R; Boyd, R (May 2013). "A systematic review of tests to predict cerebral palsy in young children". Developmental Medicine & Child Neurology. 55 (5): 418–26. doi:10.1111/dmcn.12140. PMID 23574478.
- 1 2 3 Lungu, Codrin; Hirtz, Deborah; Damiano, Diane; Gross, Paul; Mink, Jonathan W. (20 September 2016). "Report of a workshop on research gaps in the treatment of cerebral palsy". Neurology. 87 (12): 1293–1298. doi:10.1212/WNL.0000000000003116. PMC 5035982. PMID 27558377.
- 1 2 3 4 National Guideline Alliance (UK) (January 2017). Cerebral Palsy in Under 25s: Assessment and Management (PDF). London: National Institute for Health and Care Excellence (UK). ISBN 978-1-4731-2272-7. Archived (PDF) from the original on 10 September 2017. Retrieved 5 February 2017.
- ↑ Graham, David; Paget, Simon P; Wimalasundera, Neil (February 2019). "Current thinking in the health care management of children with cerebral palsy". Medical Journal of Australia. 210 (3): 129–135. doi:10.5694/mja2.12106. PMID 30739332.
- ↑ Kolawole TM, Patel PJ, Mahdi AH (1989). "Computed tomographic (CT) scans in cerebral palsy (CP)". Pediatric Radiology. 20 (1–2): 23–27. doi:10.1007/BF02010628. PMID 2602010.
- ↑ Ashwal S; Russman BS; Blasco PA; et al. (2004). "Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society". Neurology. 62 (6): 851–63. doi:10.1212/01.WNL.0000117981.35364.1B. PMID 15037681.
- 1 2 3 Rethlefsen, SA.; Ryan, DD.; Kay, RM. (October 2010). "Classification systems in cerebral palsy". Orthop Clin North Am. 41 (4): 457–67. doi:10.1016/j.ocl.2010.06.005. PMID 20868878.
- 1 2 3 4 Trabacca, Antonio; Vespino, Teresa; Di Liddo, Antonella; Russo, Luigi (September 2016). "Multidisciplinary rehabilitation for patients with cerebral palsy: improving long-term care". Journal of Multidisciplinary Healthcare. 9: 455–462. doi:10.2147/JMDH.S88782. PMC 5036581. PMID 27703369.
- 1 2 3 Becher, JG (2002). "Pediatric rehabilitation in children with cerebral palsy: general management, classification of motor disorders". American Academy of Orthotists and Prosthetists. 14 (4): 143–149. doi:10.1097/00008526-200212000-00004. Archived from the original on 2 February 2017. Retrieved 29 January 2017.
- 1 2 3 4 O’Shea, TM (2008). "Diagnosis, Treatment, and Prevention of Cerebral Palsy". Clinical Obstet Gynecol. 51 (4): 816–28. doi:10.1097/GRF.0b013e3181870ba7. PMC 3051278. PMID 18981805.
- 1 2 "Spastic cerebral palsy". Cerebral Palsy Alliance. 18 November 2015. Archived from the original on 23 March 2020. Retrieved 4 March 2020.
- ↑ Knierim, James (2020). "Chapter 3: The motor cortex". Neuroscience online: An electronic textbook of the neurosciences from the University of Texas at Houston. Archived from the original on 17 December 2019. Retrieved 4 March 2020.
- ↑ CDC (18 April 2018). "What is Cerebral Palsy? | CDC". Centers for Disease Control and Prevention. Archived from the original on 14 September 2019. Retrieved 5 March 2020.
- ↑ Nolan, Karen W.; Cole, Lynn L.; Liptak, Gregory S. (April 2006). "Use of Botulinum Toxin Type A in Children with Cerebral Palsy". Physical Therapy. 86 (4): 573–584. doi:10.1093/ptj/86.4.573 – via Academic Search Premier.
- ↑ McHale, DP; Jackson, A P; Campbell, D A; Levene, M I; Corry, P; Woods, C G; Lench, N J; Mueller, R F; Markham, A F (2000). "A gene for ataxic cerebral palsy maps to chromosome 9p12-q12". European Journal of Human Genetics. 8 (4): 267–272. doi:10.1038/sj.ejhg.5200445. PMID 10854109.
- ↑ Cheney, PD (1997). "Pathophysiology of the corticospinal system and basal ganglia in cerebral palsy". Mental Retardation and Developmental Disabilities Research Reviews. 3 (2): 153–167. doi:10.1002/(SICI)1098-2779(1997)3:2<153::AID-MRDD7>3.0.CO;2-S.
- ↑ Straub, Kathryn.; Obrzut, John E. (2009). "Effects of cerebral palsy on neurophsyological function". Journal of Developmental and Physical Disabilities. 21 (2): 153–167. doi:10.1007/s10882-009-9130-3.
- ↑ Hou, M; Zhao, J; Yu, R (2006). "Recent advances in dyskinetic cerebral palsy" (PDF). World J Pediatr. 2 (1): 23–28. Archived (PDF) from the original on 4 March 2016.
- ↑ "Athetoid Dyskinetic". Swope, Rodante P.A. Archived from the original on 2 July 2013. Retrieved 31 October 2012.
- ↑ Krägeloh-Mann, I; Horber, V (2007). "The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review". Developmental Medicine & Child Neurology. 49 (2): 144–151. doi:10.1111/j.1469-8749.2007.00144.x. ISSN 0012-1622. PMID 17254004.
- ↑ Jones, MW; Morgan, E; Shelton, JE (2007). "Cerebral palsy: introduction and diagnosis (part I)". Journal of Pediatric Health Care. 21 (3): 146–152. doi:10.1016/j.pedhc.2006.06.007. PMID 17478303.
- 1 2 Shepherd, Emily; Middleton, Philippa; Makrides, Maria; McIntyre, Sarah; Badawi, Nadia; Crowther, Caroline A (25 October 2016). "Neonatal interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews". Cochrane Database of Systematic Reviews. 2016 (10): CD012409. doi:10.1002/14651858.CD012409. PMC 6457912. PMID 29926474.
- 1 2 Sartwelle, Thomas P.; Johnston, James C. (June 2015). "Cerebral Palsy Litigation". Journal of Child Neurology. 30 (7): 828–841. doi:10.1177/0883073814543306. PMC 4431995. PMID 25183322.
- ↑ Crowther, CA; Middleton, PF; Voysey, M; Askie, L; Duley, L; Pryde, PG; Marret, S; Doyle, LW; AMICABLE, Group. (October 2017). "Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis". PLOS Medicine. 14 (10): e1002398. doi:10.1371/journal.pmed.1002398. PMC 5627896. PMID 28976987.
- ↑ Nguyen, TM; Crowther, CA; Wilkinson, D; Bain, E (28 February 2013). "Magnesium sulphate for women at term for neuroprotection of the fetus". The Cochrane Database of Systematic Reviews. 2 (2): CD009395. doi:10.1002/14651858.cd009395.pub2. PMID 23450601.
- ↑ Zeng, Xianling; Xue, Yan; Tian, Quan; Sun, Rong; An, Ruifang (January 2016). "Effects and Safety of Magnesium Sulfate on Neuroprotection". Medicine. 95 (1): e2451. doi:10.1097/MD.0000000000002451. PMC 4706271. PMID 26735551.
- 1 2 Shih, Sophy T F; Tonmukayakul, Utsana; Imms, Christine; Reddihough, Dinah; Graham, H Kerr; Cox, Liz; Carter, Rob (10 January 2018). "Economic evaluation and cost of interventions for cerebral palsy: a systematic review". Developmental Medicine & Child Neurology. 60 (6): 543–558. doi:10.1111/dmcn.13653. PMID 29319155.
- ↑ Atik, Anzari; Harding, Richard; De Matteo, Robert; Kondos-Devcic, Delphi; Cheong, Jeanie; Doyle, Lex W.; Tolcos, Mary (January 2017). "Caffeine for apnea of prematurity: Effects on the developing brain". NeuroToxicology. 58: 94–102. doi:10.1016/j.neuro.2016.11.012. PMID 27899304.
- 1 2 3 Shepherd, Emily; Salam, Rehana A; Middleton, Philippa; Makrides, Maria; McIntyre, Sarah; Badawi, Nadia; Crowther, Caroline A (8 August 2017). "Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews". Cochrane Database of Systematic Reviews. 8: CD012077. doi:10.1002/14651858.CD012077.pub2. PMC 6483544. PMID 28786098.
- ↑ Chang, Eugene (20 January 2015). "Preterm birth and the role of neuroprotection". BMJ. 350: g6661. doi:10.1136/bmj.g6661. ISSN 1756-1833. PMID 25646630. Archived from the original on 24 February 2021. Retrieved 1 August 2020.
- ↑ Jacobs, SE; Berg, M; Hunt, R; Tarnow-Mordi, WO; Inder, TE; Davis, PG (31 January 2013). "Cooling for newborns with hypoxic ischaemic encephalopathy". The Cochrane Database of Systematic Reviews. 1 (1): CD003311. doi:10.1002/14651858.CD003311.pub3. PMC 7003568. PMID 23440789.
- 1 2 3 4 5 Novak, Iona; Mcintyre, Sarah; Morgan, Catherine; Campbell, Lanie; Dark, Leigha; Morton, Natalie; Stumbles, Elise; Wilson, Salli-Ann; Goldsmith, Shona (October 2013). "A systematic review of interventions for children with cerebral palsy: state of the evidence". Developmental Medicine & Child Neurology. 55 (10): 885–910. doi:10.1111/dmcn.12246. PMID 23962350.
- ↑ "Cerebral palsy – Treatment". www.nhs.uk. NHS Choices. Archived from the original on 6 February 2017. Retrieved 6 February 2017.
- ↑ Pennington L, Goldbart J, Marshall J (2004). "Speech and language therapy to improve the communication skills of children with cerebral palsy". Cochrane Database Syst Rev (2): CD003466. doi:10.1002/14651858.CD003466.pub2. PMID 15106204.
- ↑ Jenks KM, de Moor J, van Lieshout EC, Maathuis KG, Keus I, Gorter JW (2007). "The effect of cerebral palsy on arithmetic accuracy is mediated by working memory, intelligence, early numeracy, and instruction time". Dev Neuropsychol. 32 (3): 861–79. doi:10.1080/87565640701539758. hdl:1871/34092. PMID 17956186. Archived from the original on 28 August 2021. Retrieved 1 August 2020.
- 1 2 Zaffuto-Sforza, Celeste D. (February 2005). "Aging with cerebral palsy". Physical Medicine and Rehabilitation Clinics of North America. 16 (1): 235–249. doi:10.1016/j.pmr.2004.06.014. PMID 15561553.
- ↑ Wiegerink, Diana; Roebroeck, Marij; Bender, Jim; Stam, Henk; Cohen-Kettenis, Peggy; Transition Research Group South West Netherlands (June 2011). "Sexuality of Young Adults with Cerebral Palsy: Experienced Limitations and Needs". Sexuality and Disability. 29 (2): 119–128. doi:10.1007/s11195-010-9180-6. PMC 3093545. PMID 21660090.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (help) - ↑ Hutton, Jane L. (June 2006). "Cerebral Palsy Life Expectancy". Clinics in Perinatology. 33 (2): 545–555. doi:10.1016/j.clp.2006.03.016. PMID 16765736.
- ↑ Strauss D, Brooks J, Rosenbloom R, Shavelle R (2008). "Life Expectancy in cerebral palsy: an update". Developmental Medicine & Child Neurology. 50 (7): 487–493. doi:10.1111/j.1469-8749.2008.03000.x. PMID 18611196.
- ↑ Day, Steven M; Reynolds, Robert J; Kush, Scott J (December 2015). "Extrapolating published survival curves to obtain evidence-based estimates of life expectancy in cerebral palsy". Developmental Medicine & Child Neurology. 57 (12): 1105–1118. doi:10.1111/dmcn.12849. PMID 26174088.
- ↑ Australian Bureau of Statistics 2014, 3303.0 - Causes of Death, Australia - 1. Underlying causes of death (Australia), 2014, data cube: Excel spreadsheet, http://abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3303.02014?OpenDocument Archived 12 September 2016 at the Wayback Machine
- 1 2 Kent, Ruth M. (2012). "Cerebral palsy". In Barnes, Michael; Good, David (eds.). Neurological Rehabilitation Handbook of Clinical Neurology. Oxford: Elsevier Science. pp. 443–459. ISBN 9780444595843.
- ↑ Van Zelst, B.; Miller, M.; Russo, R.; Murchland, S.; Crotty, M. (2006). "Activities of daily living in children with hemiplegic cerebral palsy: a cross-sectional evaluation using the Assessment of Motor and Process Skills". Developmental Medicine & Child Neurology. 48 (9): 723–7. doi:10.1017/S0012162206001551. PMID 16904017.
- ↑ Nieuwenhuijsen, C.; Donkervoort, M.; Nieuwstraten, W.; Stam, H.J.; Roebroeck, M.E.; Transition Research Group South West Netherlands (2009). "Experienced problems of young adults with cerebral palsy: targets for rehabilitation care". Archives of Physical Medicine and Rehabilitation. 90 (11): 1891–1897. doi:10.1016/j.apmr.2009.06.014. PMID 19887214.
- ↑ Arnould, C.; Penta, M.; Thonnard, J. (2008). "Hand impairments and their relationship with manual ability in children with cerebral palsy". Journal of Rehabilitation Medicine. 39 (9): 708–714. doi:10.2340/16501977-0111. PMID 17999009.
- ↑ Australian Institute of Health and Welfare (AIHW) 2006. Therapy and equipment needs of people with cerebral palsy and like disabilities in Australia. Archived 30 March 2015 at the Wayback Machine Disability Series. Cat. no. DIS 49. Canberra: AIHW.
- ↑ "Patients & Clients: Learn About Occupational Therapy". The American Occupational Therapy Association, Inc. Archived from the original on 26 July 2019. Retrieved 3 September 2019.
- ↑ Fedrizzi E, Pagliano E, Andreucci E, Oleari G (2003). "Hand function in children with hemiplegic cerebral palsy: prospective follow-up and functional outcome in adolescence". Dev. Med. Child Neurol. 45 (2): 85–91. doi:10.1017/s0012162203000173. PMID 12578233.
- ↑ Blesedell CE, Cohn ES, Schell AB. Willard and Spackman's occupational therapy. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. p. 705-709.
- 1 2 3 Townsend E, Stanton S, Law M. Enabling occupation: An occupational therapy perspective. Canadian Association of Occupational Therapists Ottawa, ON, Canada; 1997/2002. p. 34.
- ↑ Parham, LD; Primeau, LA (2008). "Play and occupational therapy". In Parham, LD; Fazio, LS (eds.). Play in occupational therapy for children (2nd ed.). St. Louis, Mo.: Mosby Elsevier. ISBN 978-0-323-02954-4. Archived from the original on 15 April 2015.
- ↑ Miller, S; Reid, D (December 2003). "Doing play: competency, control, and expression". Cyberpsychology & Behavior. 6 (6): 623–32. doi:10.1089/109493103322725397. PMID 14756927.
- ↑ Okimoto AM, Bundy A, Hanzlik J (2000). "Playfulness in children with and without disability: measurement and intervention". Am. J. Occup. Ther. 54 (1): 73–82. doi:10.5014/ajot.54.1.73. PMID 10686630.
- ↑ Hestenes LL, Carroll DE (2000). "The play interactions of young children with and without disabilities: Individual and environmental influences". Early Childhood Research Quarterly. 15 (2): 229–246. doi:10.1016/s0885-2006(00)00052-1.
- 1 2 3 4 Missiuna, C; Pollock N (October 1991). "Play deprivation in children with physical disabilities: the role of the occupational therapist in preventing secondary disability". American Journal of Occupational Therapy. 45 (10): 882–8. doi:10.5014/ajot.45.10.882. PMID 1835302.
- ↑ Howard, L (December 1996). "A Comparison of Leisure-Time Activities between Able-Bodied Children and Children with Physical Disabilities". British Journal of Occupational Therapy. 59 (12): 570–574. doi:10.1177/030802269605901208.
- ↑ Rigby, Patricia; Schwellnus, Heidi (January 1999). "Occupational Therapy Decision Making Guidelines for Problems in Written Productivity". Physical & Occupational Therapy in Pediatrics. 19 (1): 5–27. doi:10.1080/J006v19n01_02.
- ↑ Smith M, Sandberg AD, Larsson M (2009). "Reading and spelling in children with severe speech and physical impairments: a comparative study". International Journal of Language & Communication Disorders. 44 (6): 864–882. doi:10.1080/13682820802389873. PMID 19105069.
- 1 2 Pruitt, DW; Tsai, T (August 2009). "Common medical comorbidities associated with cerebral palsy". Physical Medicine and Rehabilitation Clinics of North America. 20 (3): 453–67. doi:10.1016/j.pmr.2009.06.002. PMID 19643347.
- ↑ Cassidy, Tony (March 1996). "All work and no play: A focus on leisure time as a means for promoting health". Counselling Psychology Quarterly. 9 (1): 77–90. doi:10.1080/09515079608256354.
- ↑ Carlon, Stacey L.; Taylor, Nicholas F.; Dodd, Karen J.; Shields, Nora (17 October 2012). "Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review". Disability and Rehabilitation. 35 (8): 647–655. doi:10.3109/09638288.2012.715721. PMID 23072296.
- ↑ Verschuren, Olaf; Peterson, Mark D; Balemans, Astrid C J; Hurvitz, Edward A (August 2016). "Exercise and physical activity recommendations for people with cerebral palsy". Developmental Medicine & Child Neurology. 58 (8): 798–808. doi:10.1111/dmcn.13053. PMC 4942358. PMID 26853808.
- ↑ Bloemen, Manon; Van Wely, Leontien; Mollema, Jurgen; Dallmeijer, Annet; de Groot, Janke (March 2017). "Evidence for increasing physical activity in children with physical disabilities: a systematic review". Developmental Medicine & Child Neurology. 59 (10): 1004–1010. doi:10.1111/dmcn.13422. PMID 28374442.

- ↑ Bult, M.K.; Verschuren, O.; Jongmans, M.J.; Lindeman, E.; Ketelaar, M. (September 2011). "What influences participation in leisure activities of children and youth with physical disabilities? A systematic review". Research in Developmental Disabilities. 32 (5): 1521–1529. doi:10.1016/j.ridd.2011.01.045. PMID 21388783.
- ↑ Coleman, Nailah; Nemeth, Blaise A.; LeBlanc, Claire M. A. (December 2018). "Increasing Wellness Through Physical Activity in Children With Chronic Disease and Disability". Current Sports Medicine Reports. 17 (12): 425–432. doi:10.1249/JSR.0000000000000548. ISSN 1537-8918. PMID 30531459.
- 1 2 King, G; Law, M; King, S; Rosenbaum, P; Kertoy, MK; Young, NL (2003). "A conceptual model of the factors affecting the recreation and leisure participation of children with disabilities". Physical & Occupational Therapy in Pediatrics. 23 (1): 63–90. doi:10.1080/J006v23n01_05. PMID 12703385.
- 1 2 3 Aitchison, Cara (December 2003). "From leisure and disability to disability leisure: developing data, definitions and discourses". Disability & Society. 18 (7): 955–969. doi:10.1080/0968759032000127353. Archived from the original on 9 September 2021. Retrieved 1 August 2020.
- ↑ Imms, C (2008). "Children with cerebral palsy participate: a review of the literature". Disability and Rehabilitation. 30 (24): 1867–84. doi:10.1080/09638280701673542. PMID 19037780.
- 1 2 3 Specht J, King G, Brown E, Foris C (2002). "The importance of leisure in the lives of persons with congenital physical disabilities". Am. J. Occup. Ther. 56 (4): 436–445. doi:10.5014/ajot.56.4.436. PMID 12125833.
- ↑ Frisch, Dana; Msall, Michael E. (August 2013). "Health, functioning, and participation of adolescents and adults with cerebral palsy: A review of outcomes research". Developmental Disabilities Research Reviews. 18 (1): 84–94. doi:10.1002/ddrr.1131. PMID 23949832.
- ↑ Lawrence, Hillary; Hills, Sara; Kline, Nicole; Weems, Kyra; Doty, Antonette (November 2016). "Effectiveness of Exercise on Functional Mobility in Adults with Cerebral Palsy: A Systematic Review". Physiotherapy Canada. 68 (4): 398–407. doi:10.3138/ptc.2015-38LHC. PMC 5125497. PMID 27904240.
- ↑ Bagatell, Nancy; Chan, Dara; Rauch, Kimberly Karrat; Thorpe, Deborah (January 2017). ""Thrust into adulthood": Transition experiences of young adults with cerebral palsy". Disability and Health Journal. 10 (1): 80–86. doi:10.1016/j.dhjo.2016.09.008. PMID 27756560.
- ↑ Turk, M.A., Overeynder, J.C., & Janicki, M.P. (Eds.). (1995). Uncertain Future – Aging and Cerebral Palsy: Clinical Concerns. Albany: New York State Developmental Disabilities Planning Council. Archived 3 August 2016 at the Wayback Machine
- ↑ Morgan, P.; McGinley, J. (17 April 2013). "Gait function and decline in adults with cerebral palsy: a systematic review". Disability and Rehabilitation. 36 (1): 1–9. doi:10.3109/09638288.2013.775359. PMID 23594053.
- ↑ Peterson, M. D.; Gordon, P. M.; Hurvitz, E. A. (February 2013). "Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour" (PDF). Obesity Reviews. 14 (2): 171–182. doi:10.1111/j.1467-789X.2012.01052.x. hdl:2027.42/96337. PMID 23094988. Archived from the original on 28 August 2021. Retrieved 1 August 2020.
- ↑ "CP and ageing". Scope. Archived from the original on 6 May 2016. Retrieved 24 December 2016.
- 1 2 KEMBHAVI, GAYATRI; DARRAH, JOHANNA; PAYNE, KELSEY; PLESUK, DEVON (July 2011). "Adults with a diagnosis of cerebral palsy: a mapping review of long-term outcomes". Developmental Medicine & Child Neurology. 53 (7): 610–614. doi:10.1111/j.1469-8749.2011.03914.x. PMID 21418196.
- ↑ Horsman, M.; Suto, M.; Dudgeon, B.; Harris, S. R. (22 February 2010). "Ageing with cerebral palsy: psychosocial issues". Age and Ageing. 39 (3): 294–299. doi:10.1093/ageing/afq018. PMID 20178997.
- ↑ Odding, E; Roebroeck, ME; Stam, HJ (28 February 2006). "The epidemiology of cerebral palsy: incidence, impairments and risk factors". Disability and Rehabilitation. 28 (4): 183–91. doi:10.1080/09638280500158422. PMID 16467053.
- ↑ Johnson, Ann (2002). "Prevalence and characteristics of children with cerebral palsy in Europe". Developmental Medicine & Child Neurology. 44 (9): 633–40. doi:10.1111/j.1469-8749.2002.tb00848.x. PMID 12227618.
- ↑ Dalembert, George; Brosco, Jeffrey P. (June 2013). "Do Politics Affect Prevalence? An Overview and the Case of Cerebral Palsy". Journal of Developmental & Behavioral Pediatrics. 34 (5): 369–374. doi:10.1097/DBP.0b013e31829455d8. PMID 23751888.
- ↑ definition and classification of cerebral palsy.pdf "Proposed definition and classification of cerebral palsy, April 2005" (PDF). Developmental Medicine & Child Neurology. 47 (8): 571. 2007. doi:10.1111/j.1469-8749.2005.tb01195.x.
{{cite journal}}: Check|url=value (help) - ↑ Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R (2007). "How common are the "common" neurologic disorders?". Neurology. 68 (5): 326–337. doi:10.1212/01.wnl.0000252807.38124.a3. PMID 17261678.
- ↑ "The Definition and Classification of Cerebral Palsy". Developmental Medicine & Child Neurology. 49: 1–44. 28 June 2008. doi:10.1111/j.1469-8749.2007.00201.x.
- ↑ Access Economics (2008) The Economic Impact of Cerebral Palsy in Australia in 2007. Access Economics, Canberra, ACTArchived 21 November 2008 at the Wayback Machine
- ↑ Centers for Disease Control and Prevention (CDC) (2004). "Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment—United States, 2003". Morbidity and Mortality Weekly Report. 53 (3): 57–9. PMID 14749614. Archived from the original on 19 September 2007. Retrieved 12 August 2007.
- ↑ "Medicaid.gov: Self Directed Services". Medicaid. Archived from the original on 19 June 2017. Retrieved 19 June 2017.
- ↑ "NIRAMAYA Ministry of Social Justice and Empowerment (MSJE)". thenationaltrust.gov.in. Archived from the original on 27 February 2017. Retrieved 27 February 2017.
- ↑ V, MEERA SIVA, NALINAKANTHI (29 November 2015). "Financially able". The Hindu Business Line. Archived from the original on 31 December 2019. Retrieved 1 August 2020.
- ↑ "Definition of Cerebral". Archived from the original on 28 August 2021. Retrieved 1 August 2020.
- ↑ "Bell's Palsy (Facial Nerve Problems): Symptoms, Treatment & Contagious". Archived from the original on 23 April 2008. Retrieved 1 August 2020.
- ↑ Shevell, Michael (19 December 2018). "Cerebral palsy to cerebral palsy spectrum disorder : Time for a name change?". Neurology: 10.1212/WNL.0000000000006747. doi:10.1212/WNL.0000000000006747. PMID 30568002.
- ↑ "Cerebral Palsy: a Guide for Care". Archived from the original on 17 July 2007. Retrieved 29 July 2007.
- 1 2 Brindle, David (22 May 2002). "A very telling tale". The Guardian. London. Archived from the original on 29 July 2016. Retrieved 6 December 2016.
- ↑ Zimmer, Benjamin (5 February 2007). "A brief history of "spaz"". Language Log. University of Pennsylvania. Archived from the original on 9 August 2007. Retrieved 29 July 2007.
- ↑ Erickson, Glenn (22 May 2007). "DVD Savant Review: Goodbye CP". DVDtalk. Archived from the original on 30 April 2011.
- ↑ Borah, Prabalika (28 June 2011). "Movie with a twist". The Hindu. Chennai, India. Archived from the original on 27 September 2013.
- ↑ Alawadhi, Neha (26 March 2011). "Charting unexplored territory in Indian cinema". The Hindu. Chennai, India. Archived from the original on 27 September 2013.
- ↑ Rai, Ashutosh (3 January 2012). "spandan" "Review of Marathi Film "SPANDAN"". The Newsleak.in.
- ↑ "Vegitha, carving a niche for herself". The Deccan Chronicle. 7 June 2011. Archived from the original on 9 June 2011.
- ↑ Champlin, Charles (21 December 1989). "My Left Foot". Los Angeles Times. Archived from the original on 9 September 2021. Retrieved 1 August 2020.
- ↑ "Being on Call the Midwife gave me the sense of being an actor in my own right". 16 February 2014. Archived from the original on 5 September 2015. Retrieved 12 August 2015.
- ↑ Dalene Rovenstine (20 September 2016). "Micah Fowler is the breakout star of Speechless". EntertaainmentWeekly. Archived from the original on 24 September 2016. Retrieved 3 October 2016.
- ↑ Kacala, Alexander (12 April 2019). "'Special': Groundbreaking new Netflix series stars gay man with cerebral palsy". NBC News. Archived from the original on 28 April 2019. Retrieved 22 April 2019.
- ↑ Saxon, Wolfgang (7 September 1997). "Harold E. Yuker, 73, Authority On Attitudes Toward Disabled". The New York Times. New York: NYTC. ISSN 0362-4331. Archived from the original on 12 April 2012. Retrieved 30 July 2012.
- ↑ "Neil Young". Biography. Archived from the original on 8 October 2019. Retrieved 17 October 2019.
- ↑ "Neil Young Files for Divorce From Pegi Young". Rolling Stone. 26 August 2014. Archived from the original on 5 November 2019. Retrieved 17 October 2019.
- ↑ Michaels, Sean (27 August 2014). "Neil Young files for divorce from Pegi, his wife of 36 years". The Guardian. ISSN 0261-3077. Archived from the original on 28 August 2021. Retrieved 17 October 2019.
- ↑ "Comedian Geri Jewell headlines diversity event". WMU News. 16 May 2002. Archived from the original on 7 March 2012. Retrieved 6 December 2010.
- ↑ "Josh Blue wins "Last Comic Standing"". Archived from the original on 28 June 2010. Retrieved 6 December 2010.
- ↑ ""Last Comic Standing" biography". Archived from the original on 13 September 2010. Retrieved 6 December 2010.
- ↑ "Jason Benetti Is Voice of Hope in Face of Cerebral Palsy". Archived from the original on 17 March 2013.
- ↑ "Britain's Got Talent: Jack Carroll to coin it with TV show, DVD and book deals Archived 2 August 2013 at the Wayback Machine," The Mirror, accessed 1 August 2013.
- ↑ "Pageant Is Her Crowning Achievement: Miss Iowa, In Las Vegas For Miss USA Pageant, Was Born With Cerebral Palsy". Las Vegas: CBS. 10 April 2008. Archived from the original on 6 November 2008. Retrieved 5 June 2010.
- ↑ Tim Lewis (7 August 2011). "Comedian Francesca Martinez: "Surely I can't be more unfunny than Patsy Palmer"". The Guardian. London. Archived from the original on 3 February 2013.
- ↑ "O'Hanlon heads to Melbourne in career-best form". Athletics Australia. 1 March 2012. Archived from the original on 24 April 2012. Retrieved 18 April 2012.
- ↑ "HarperCollins Publishers India Ltd". Archived from the original on 11 June 2011.
- ↑ Franks, Tim (7 July 2008). "Jerusalem Diary: Monday 7 July". BBC News. Archived from the original on 11 May 2009.
- ↑ Heydarpour, Roja. "The Comic Is Palestinian, the Jokes Bawdy" Archived 21 November 2012 at the Wayback Machine, The New York Times, 21 November 2006. Accessed 22 March 2011. "Ms. Zayid, who has a home in Cliffside Park, N.J., recently returned from Hollywood, where she lived while working on developing her one-woman show."
- ↑ IMEU (18 October 2013). "Maysoon Zayid: Actor and Comedian". Institute for Middle East Understanding. Archived from the original on 23 December 2016. Retrieved 8 December 2016.
- ↑ "Celebrity Ambassadors". Archived from the original on 9 June 2015. Retrieved 18 June 2015.
- ↑ "REVIEW: Zach Anner's If at Birth You Don't Succeed Is an Inspiring, Hilarious Memoir About One Man's Life with Cerebral Palsy". People. 11 March 2016. Archived from the original on 15 February 2018. Retrieved 6 December 2016.
- ↑ ABS Staff (4 June 2014). "10 Celebrities With Physical Deformities You May Have Never Noticed". Atlanta Black Star. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ↑ "IPC Athletics Records". www.paralympic.org. Archived from the original on 10 September 2012. Retrieved 10 September 2012.
- ↑ "Paralympics 2012: Cockroft wins first GB track gold". BBC Sport. 31 August 2012. Archived from the original on 1 September 2012. Retrieved 1 September 2012.
- ↑ "Paralympics 2012: Hannah Cockroft wins second sprint gold". BBC Sport. 6 September 2012. Archived from the original on 8 September 2012. Retrieved 6 September 2012.
- ↑ Reviews, L. J. "Perspectives on Disability". Library Journal. Archived from the original on 30 September 2020. Retrieved 29 April 2020.
- ↑ "Cerebral Palsy Litigation". Radio National. 10 October 2005. Archived from the original on 22 February 2017. Retrieved 21 February 2017.
External links
| Look up cerebral palsy in Wiktionary, the free dictionary. |
| Library resources about Cerebral palsy |
- Cerebral palsy at Curlie
- Cerebral Palsy Information Page Archived 18 March 2021 at the Wayback Machine at NINDS
| Classification | |
|---|---|
| External resources |